Cell-based immunotherapy is a targeted approach to cancer treatment that continues to evolve beyond its early successes treating leukemia. Researchers employ new technologies, such as chimeric antigen receptors (CARs) expressed on the immune cell surface, to effectively target hematological malignancies and solid tumors with cell therapy.1,2
Developing Safe and Effective CARs
To develop CARs, scientists engineer hybrid molecules made of T cell receptors and antibody fragments. These receptors direct immune cells to recognize and destroy cancer cells that express a specific antigen targeted by the CAR antibody domain. In recent years, scientists fine tuned CAR therapies for improved safety and efficacy. Optimized CAR molecules have high target antigen specificity, enable efficient immune cell activation, and cause minimal off-target effects.3,4
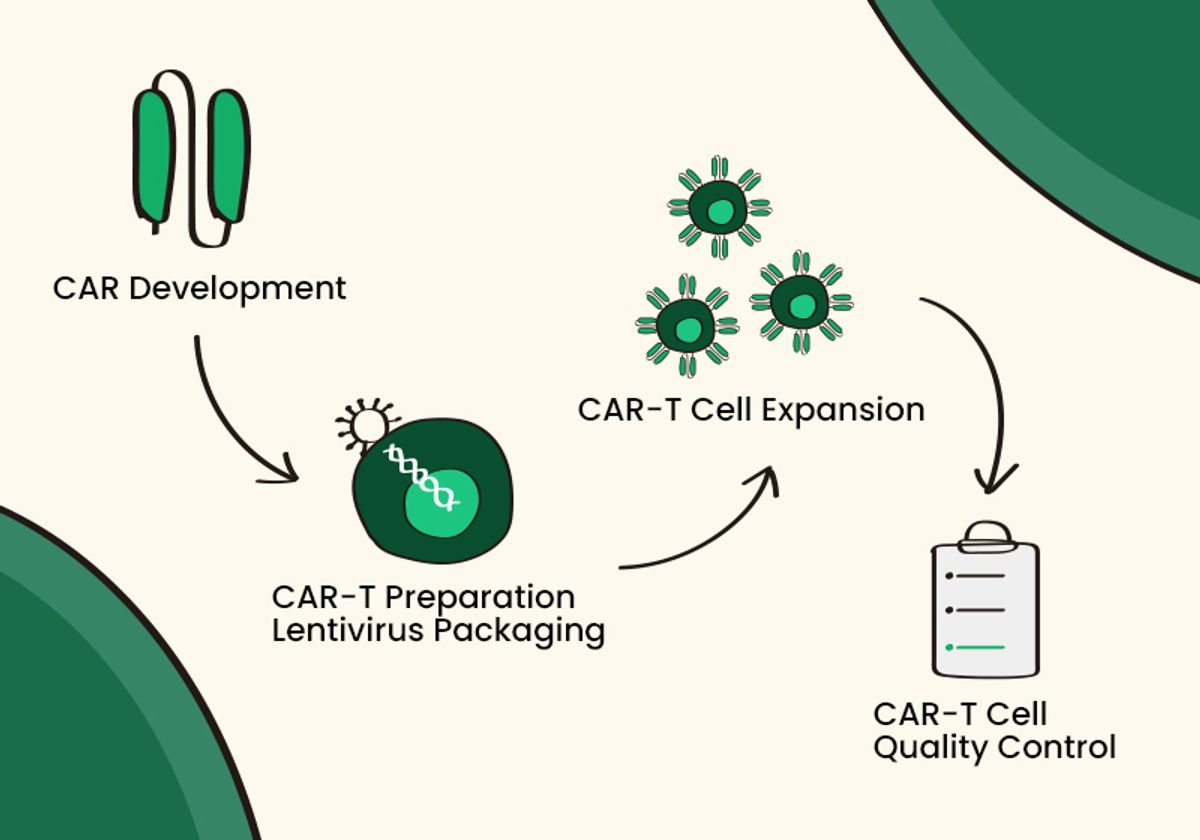
Once an optimal CAR is developed, researchers use reliable lentiviral packaging methods to transfer it to immune cells for CAR expression and quality control experiments such as immunoassays and cell viability assays. CAR-T therapies, T cells equipped with CAR expression, continue to lead the way in cell-based therapeutics. Therefore, researchers must design CAR molecules in ways that ensure the durability and persistence of CAR-expressing immune cells in the body.2-6
Putting the CAR in Cellular Therapy
For CAR-T cell therapy, researchers genetically engineer T cells derived from either a patient or a donor, modify them to express CARs, expand the cells ex vivo, and infuse them into a patient’s bloodstream. Since 2017, over 700 CAR-T therapy clinical trials have been registered and the FDA has approved six CAR-T therapies for the treating hematological malignancies, including leukemia, lymphoma, and multiple myeloma.1 However, despite their clinical significance, there are limitations to producing and employing CAR-T therapies. Collecting T cells from a patient or donor is costly and time-consuming, especially for patients with compromised immune systems. Additionally, CAR-T cell therapy may cause severe side effects such as cytokine release syndrome (CRS) or neurotoxicity. Finally, CAR-T cells struggle to penetrate the tumor microenvironment (TME), which can hinder their therapeutic function.3,4
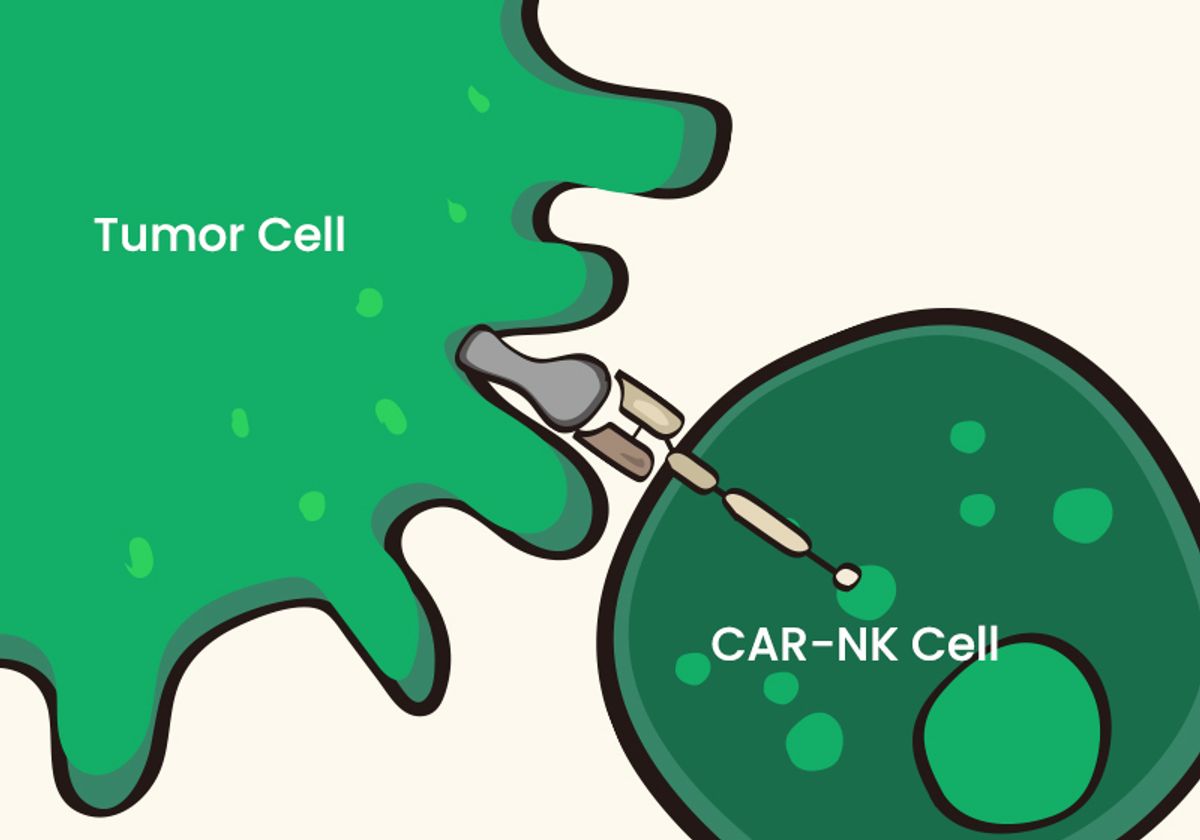
To overcome conventional CAR-T limitations, researchers turn to promising new CAR strategies. In multi-target CAR-T, scientists design CARs that recognize two or more antigens per receptor, resulting in broader immune coverage. Another new avenue involves alternative immune cell types such as natural killer (NK)-based cell therapies. Part of the innate immune response, NK cells can eliminate abnormal cells, including tumor cells and virally-infected cells. Researchers produce CAR-NK cell therapies with the same receptor-antigen hybrid technology as CAR-T, but express the CAR in NK cells. Scientists can derive NK cells from a variety of sources, including umbilical cord blood (UCB), peripheral blood (PB), human-induced pluripotent stem cells, human embryonic stem cells, hematopoietic stem cells, and NK cell lines. In comparison to CAR-T therapy, CAR-NK cell therapy is safer with a lower chance of negative immune side effects, more efficient antitumor activity, and higher efficiency for off-the-shelf production. CAR-NK cell therapies for solid tumors and hematological malignancies have shown promising results in clinical trials.1,3,6,7
Complex Development, Comprehensive Solutions
Before cell therapies reach the clinic, scientists develop them through a multistep process. Whether the therapy is CAR-T or CAR-NK, researchers must collect immune cells, employ genetic engineering to create a targeted CAR, transduce immune cells for CAR expression, culture and expand the CAR-expressing cells, and test for quality control.
As a global leading supplier of bioreagents and CRO services for the biopharmaceutical field, Sino Biological provides comprehensive solutions for scientists developing CAR-T and CAR-NK cell therapies.5,6 These solutions cover each step from CAR development to CAR-T and CAR-NK cell preparation and cell quality control. Sino Biological’s reagents and services can support researchers during each stage of cell therapy development from early target discovery to preclinical phase.
References
- A.R. Saez-Ibañez et al., “Landscape of cancer cell therapies: trends and real-world data,” Nat Rev Drug Discov, 21(9):631-32, 2022. doi: 10.1038/d41573-022-00095-1
- K. Skorka et al., “The application of CAR-T cells in haematological malignancies,” Arch Immunol Ther Exp (Warsz), 68(6):34, 2020. doi: 10.1007/s00005-020-00599-x
- “Novel Cancer Therapies Using CAR Technology: CAR Applications in Non-T Cells,” Sino Biological, https://www.sinobiological.com/resource/application-note/novel-cancer-therapies-using-car-technology, accessed April 25, 2023
- “CAR-T: A Promising Cell Therapy for Cancer Treatment,” Sino Biological, https://www.sinobiological.com/resource/application-note/car-t-a-promising-cell-therapy-for-cancer-treatment, accessed April 25, 2023
- “Comprehensive CAR-T Therapy Development Solutions,” Sino Biological, https://www.sinobiological.com/category/car-t-cell-immunotherapy, accessed April 25, 2023
- “One-stop Research Support for CAR-NK Therapy,” Sino Biological, https://www.sinobiological.com/category/solutions/car-nk-therapy, accessed April 25, 2023
- K.E. Ruppel et al., “Taking lessons from CAR-T cells and going beyond: Tailoring design and signaling for CAR-NK cells in cancer therapy,” Front Immunol, 13:822298, 2022. doi: 10.3389/fimmu.2022.822298