Stay up to date on the latest science with Brush Up Summaries.
What Is Hybridoma Technology?
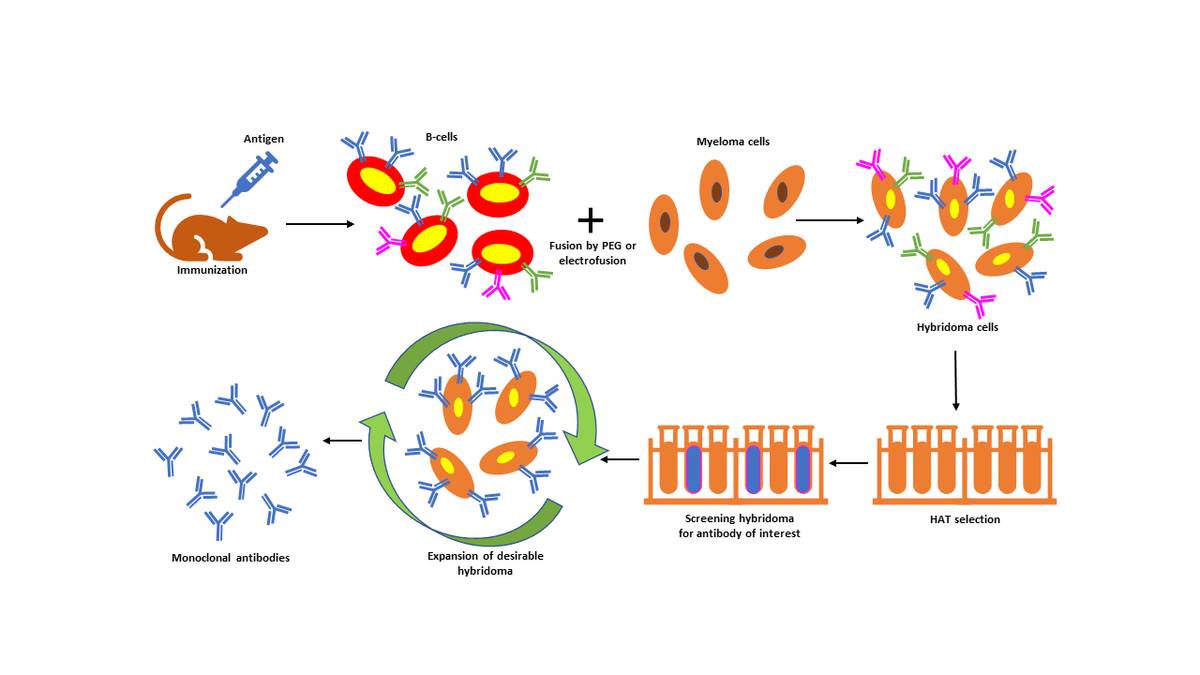
Hybridoma technology involves fusing short-lived antibody-producing B cells with immortal myeloma cells. The resultant cell lines produce a never-ending supply of a specific monoclonal antibody.1 The technique was created in 1975 by Nobel prize-winning scientists Georges Kohler and Cesar Milstein.
Monoclonal Antibodies
Scientists create monoclonal antibodies by cloning a single antibody-producing cell line. In contrast to polyclonal antibodies, monoclonals are highly specific to an antigen. By fusing myeloma cells that can divide indefinitely with B cells that produce specific antibodies against the target of interest, researchers obtain a nearly unlimited source of identical monoclonal antibodies.2
Since 1986, over 117 monoclonal antibodies have been FDA-approved, beginning with the mouse monoclonal antibody Muromonab-CD3 for kidney transplant rejection.3 In additional to antibodies from mice and humans, researcher have produced chimeric and humanized monoclonal antibodies that are composed of sequences from both species. By replacing mouse-derived protein sequences with human ones, these monoclonal antibodies reduce the risk of triggering an immune response in humans.4 Using various methods, scientists have also produced monoclonal antibodies from other mammals for diverse purposes.
Hybridoma Technology Step by Step
Step 1: Immunization
Researchers inject a mammal, typically a mouse, with a target antigen, stimulating an immune response. Antigen injection may occur in a series over the course of several weeks. Then, researchers harvest the mouse's spleen to obtain B cells that produce the desired antibody.1
Step 2: Cell Fusion
Researchers fuse antibody-producing B cells with myeloma cells in cell culture. Polyethylene glycol (PEG) facilitates fusion of both cells’ plasma membranes, forming a single hybridoma cell with two or more nuclei. Alternatively, electrofusion can merge the cells using a pulsed electrical field.5
Step 3: Hybridoma Cell Growth
Less than 1 percent of the initial cells fuse to form hybridoma cells. Unused B cells in the culture stop dividing naturally, while chemotherapy destroys the unfused myeloma cells. Researchers use HAT (hypoxanthine-aminopterin-thymidine) medium to allow the selective proliferation of immortal monoclonal antibody-producing cell lines. The aminopterin in the HAT medium stops nucleotide synthesis, while hypoxanthine and thymidine can be used by cells, such as B cells, carrying the HGPRT (hypoxanthine-guanine phosphoribosyl transferase) enzyme. Hybridoma cells with functional HGPRT enzyme can survive and grow, while myeloma cells lacking it eventually die.1
Step 4: Screening
Researchers often screen hybridoma cells for the monoclonal antibody of interest using an enzyme-linked immunosorbent assay (ELISA). Indirect ELISAs identify antibodies with the appropriate specificity by immobilizing the antigen on a surface and incubating it with a hybridoma cell supernatant. Researchers also use techniques including western blot, flow cytometry, and immunoprecipitation-mass spectrometry to screen their hybridoma cultures.1
Step 5: Hybridoma Expansion
The final step involves cloning desired hybridoma cells to obtain a stable cell population and growing the culture to collect large amounts of monoclonal antibodies. This can be achieved through one of two methods.1
- In vitro growth of hybrid cells in tissue culture
- In vivo growth following inoculation of hybridoma cells into a mouse’s abdomen
Hybridoma Benefits and Limitations
This technology offers numerous advantages, namely1,6
- Precise antigen targeting
- A never-ending supply of consistent antibodies
- High sensitivity and specificity for use in biological assays
- Elimination of the need for animal models (in vitro method)
- Utilization in therapeutic and diagnostic treatments, vaccine creation, and chemotherapy
Nevertheless, the technology also has a few limitations.1,7
- Long production time
- Resource-intensive and expensive workflow
- Not suitable for generating short peptides and fragment antigens
- Susceptibility to contamination and poor cell viability
- Risk of virus contamination and disease transmission
- Absence of stable myeloma cells for human antibody production
Hybridoma Applications
Diagnostic applications
Owing to their high specificity, the antibodies produced by hybridoma technology have a wide range of diagnostic applications, including the following.
- Enzyme-linked immunosorbent (ELISA): detecting HIV antibodies, hepatitis B surface antigen, and pregnancy hormone8
- Immunofluorescence assay (IFA): detecting autoimmune disorders, influenza virus, and Chlamydia trachomatis9
- Western blot: analyzing cancer biomarkers8
- Flow cytometry: assessing immune cells in HIV, leukemia, and lymphoma8
- Immunohistochemistry (IHC): analyzing cancer biomarkers8
- Rapid antigen tests: detecting malaria, dengue, Zika virus, and COVID-1910,11
Immunotherapy
There are various FDA-approved indications of monoclonal antibodies (see table below).3 Common indications include the following.
- Cancer treatment: anticancer immunotherapy against prostate, breast, lung, bladder, liver, gastric, colorectal, and endometrial cancer
- Autoimmune disorders: management of rheumatoid arthritis, Crohn's disease, lupus, psoriasis
- Infectious diseases: prevention and treatment of respiratory syncytial virus and COVID-19
- Organ transplant: rejection prevention of kidney, liver, lung, and heart transplants
Noteworthy FDA-approved monoclonal antibodies3
Monoclonal antibody | Antibody subtype | Target antigen | First FDA-approved indication | Year of approval |
Lecanemab | Humanized IgG1 | Amyloid beta protofibrils | Alzheimer’s disease | 2023 |
Margetuximab | Chimeric IgG1 | HER2 | HER2+ breast cancer | 2020 |
Trastuzumab | Humanized IgG1 ADC | HER2 | HER2+ breast cancer | 2019 |
Omalizumab | Humanized IgG1 | IgE | Asthma | 2003 |
Adalimumab | Human IgG1 | TNF | Rheumatoid arthritis | 2002 |
Infliximab | Chimeric IgG1 | TNF | Crohn disease | 1998 |
Basiliximab | Chimeric IgG1 | IL-2R | Prevention of kidney transplant rejection | 1998 |
Palivizumab | Humanized IgG1 | Respiratory syncytial virus | Prevention of respiratory syncytial virus infection | 1998 |
Rituximab | Chimeric IgG1 | CD20 | Non-Hodgkin lymphoma | 1997 |

References
- Parray HA, Shukla S, Samal S, et al. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int Immunopharmacol. 2020;85:106639. doi:10.1016/j.intimp.2020.106639
2. Mitra S, Tomar PC. Hybridoma technology; advancements, clinical significance, and future aspects. J Genet Eng Biotechnol. 2021;19(1):159. doi:10.1186/s43141-021-00264-6
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed March, 2023.
4. Castelli MS, McGonigle P, Hornby PJ. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect. 2019;7(6):e00535. doi:10.1002/prp2.535
5. Tabll A, Abbas AT, El-Kafrawy S, Wahid A. Monoclonal antibodies: Principles and applications of immmunodiagnosis and immunotherapy for hepatitis C virus. World J Hepatol. 2015;7(22):2369-2383. doi:10.4254/wjh.v7.i22.2369
6. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0
7. Moraes JZ, Hamaguchi B, Braggion C, et al. Hybridoma technology: is it still useful? Curr Res Immunol. 2021;2:32-40. doi:10.1016/j.crimmu.2021.03.002
8. Sundarraj S, Rajagopal G, Sundaramahalingam B, et al. Methods of Protein Detection in Cancer for Diagnosis, Prognosis and Therapy. Protein Detection. IntechOpen; 2022. doi.org/10.5772/intechopen.101050
9. Mazzulli T. Laboratory Diagnosis of Infection Due to Viruses, Chlamydia, Chlamydophila, and Mycoplasma. Principles and Practice of Pediatric Infectious Disease. 2008;1352-1368. doi:10.1016/B978-0-7020-3468-8.50293-5
10. Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel. New York: Oxford University Press; 2017.
11. Drain PK. Rapid Diagnostic Testing for SARS-CoV-2. N Engl J Med. 2022;386(3):264-272. doi:10.1056/NEJMcp2117115