A Picture Sparks a Thousand Words
A scientific image can conceal even more than it reveals. Scientists can now share their untold behind-the-image stories in our new Science Snapshot column.
Recently, Google Photos digitally prodded me to revisit a few vacation photos taken a couple of years ago. Once I clicked “relive the day,” I saw my selfie against a scenic backdrop of the calm Chicago River and sprawling high rise buildings. Interestingly, the photo reminded me of something beyond the visible picturesque surroundings: the morning of the boat ride.
I recalled how I had overslept, gotten ready in record time, and frantically biked to just make it for the tour. I took the photo to mark the triumphant moment that I boarded the boat.
The incident got me thinking about the popular saying, “A picture is worth a thousand words.” The photo substituted for my lengthy description of the tour, but I ended up narrating a different 1,000-word story to my family: the saga behind the click.
Pictures often evoke memories of equally interesting behind-the-scenes stories. Scientific images are no exception. When researchers look through their publication-worthy figures, they no doubt have a roster of incidents spring to their minds. Some might recollect the frustrating failed attempts or the tireless efforts to troubleshoot an experiment. Others might relive the emotions of joy, exhaustion, and relief that flooded in when they finally confirmed their hypotheses.
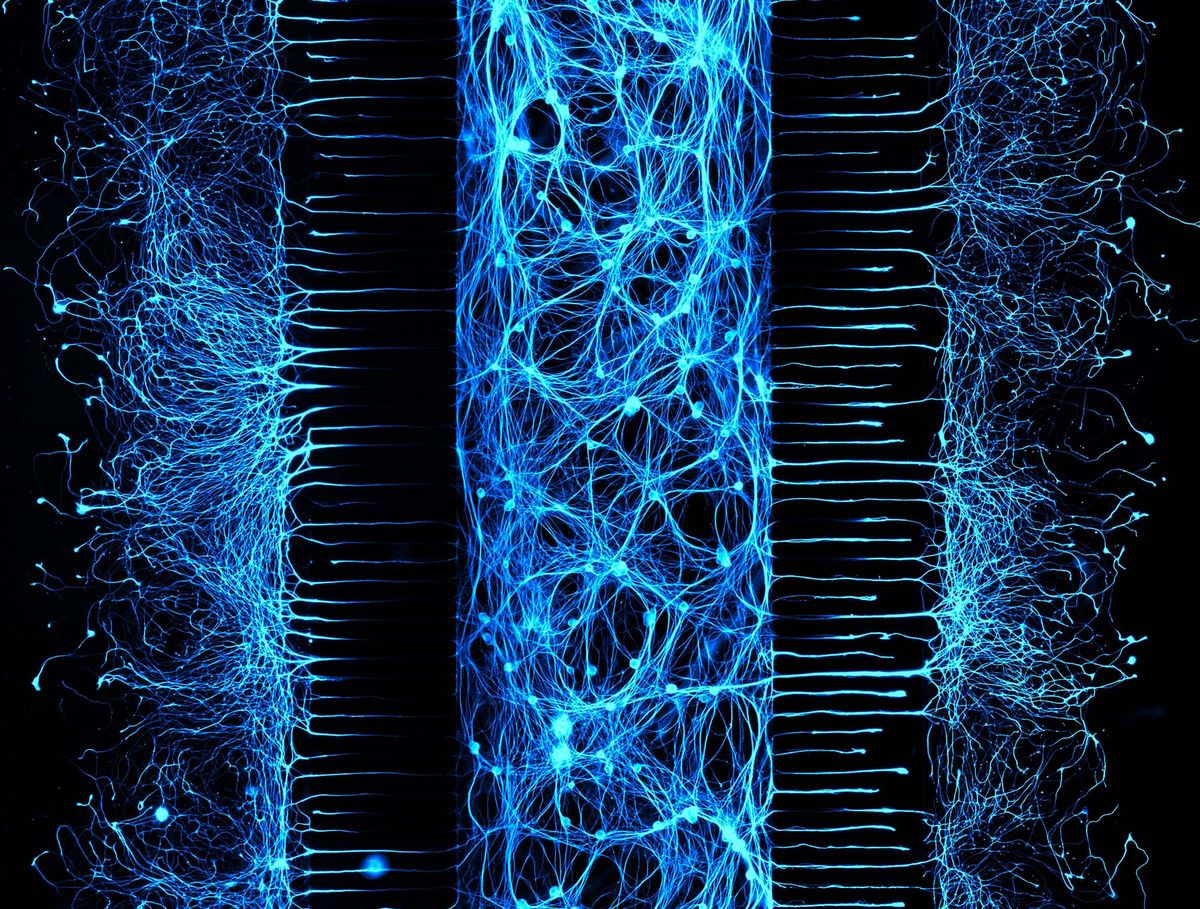
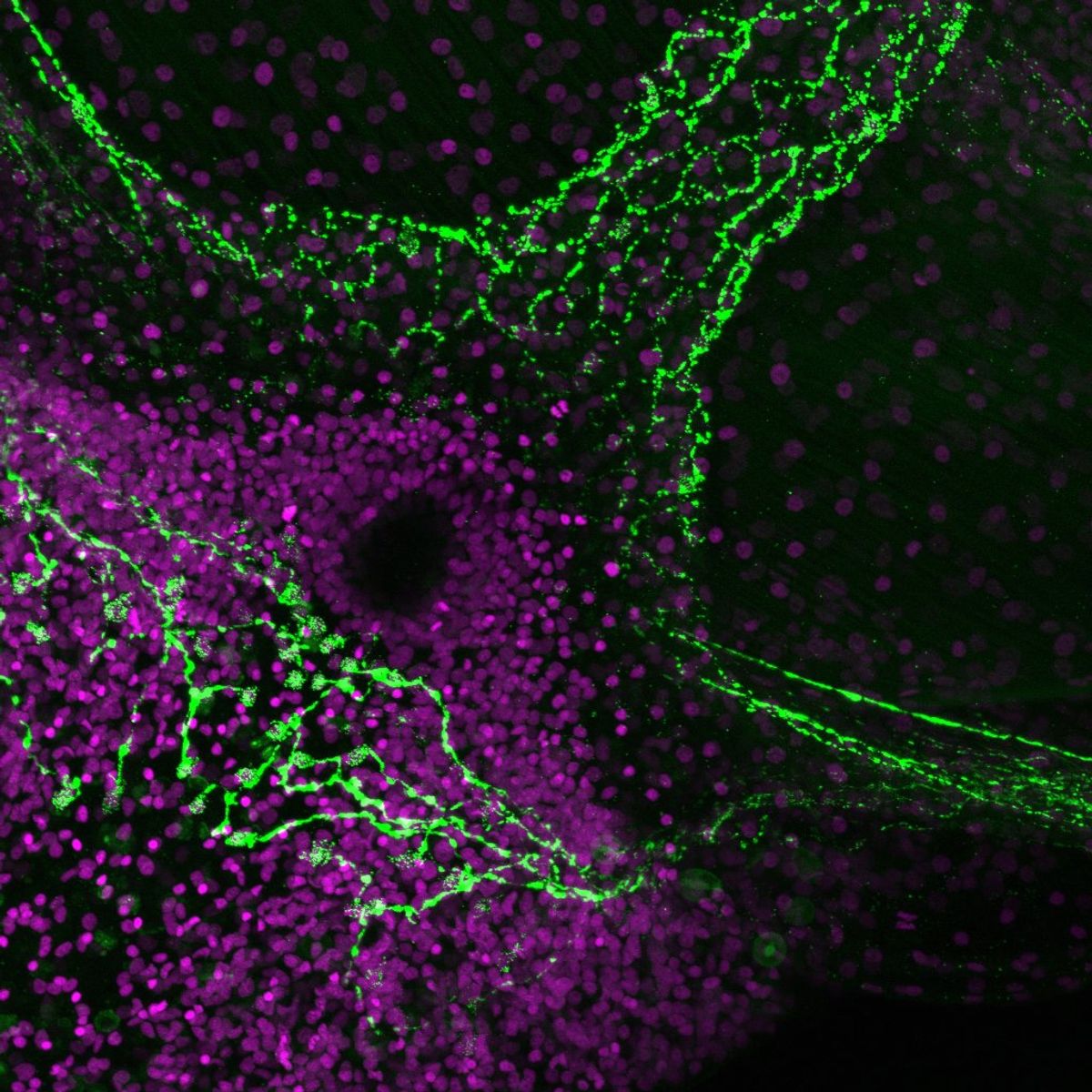
While researchers convey their motivations, experimental methods, and scientific data through papers, there is no equivalent outlet for their human experiences. As science storytellers, we at The Scientist value the behind-the-image story just as much as the data in an image. In our new Science Snapshot column, we intend to bring these scientists’ stories to the forefront. In our first one, neuroscientist Selena Romero at the University of North Carolina narrated her experience of imaging mouse peripheral neurons in a three-compartment microfluidic chamber.
Do you have a Science Snapshot story to share? Submit your feedback or story below.