Researchers use in vitro and in vivo model systems to investigate complex biological processes. However, these models present their own challenges.1 Two-dimensional cell culture is not able to demonstrate the full complexity of three-dimensional processes, including cellular interactions with extracellular matrix (ECM) proteins and neighboring cells. Moreover, cellular morphology is often modified in vitro, where cell shape and cell junction quantity can be altered. Although animal models enable scientists to study processes in vivo, their findings may not be translatable to humans and the system is not amenable to high-throughput screening.
A New Model System
To overcome the challenges of the existing model systems, researchers developed organoids, which are 3D multicellular structures. Organoids contain multiple organ-specific cell types interacting with each other and ECM proteins, thereby mimicking the functionality and structure of organs in vivo. This allows researchers to model organ-specific diseases, such as inflammatory bowel disease using gastrointestinal organoids, cystic fibrosis using lung organoids, and bladder cancer using urothelial cancer organoids.2 Additionally, they employ organoids to investigate development, drug response and toxicity, and interactions between epithelial cells and host microbiota or pathogens.
Scientists generate organoids from stem cells, where they either use induced pluripotent stem cells or extract adult stem cells from primary tissue. If they obtain stem cells from patient samples, these organoids are called patient-derived organoids (PDOs). Stem cells self-organize into organoids after researchers expose them to physical cues, such as mechanical forces exerted on the cells by ECM proteins, and biochemical cues, such as growth factors involved in proliferation, differentiation, and self-renewal.3
Problems with Existing Organoid Models
Traditional methods produce organoids with a “basolateral-out” morphology, which prevents scientists from accessing the internally located apical surface. This surface is responsible for absorbing nutrients and drugs, secreting enzymes, and interacting with microbes. To investigate these apical processes, researchers primarily employ microinjection, where they inject compounds or microorganisms into the interior cavity of the organoid.4 However, this challenging method can disrupt the organoid’s barrier and also prevents scientists from running synchronized drug exposure experiments, as they need to inject each organoid sequentially.
Reversing Organoid Polarity
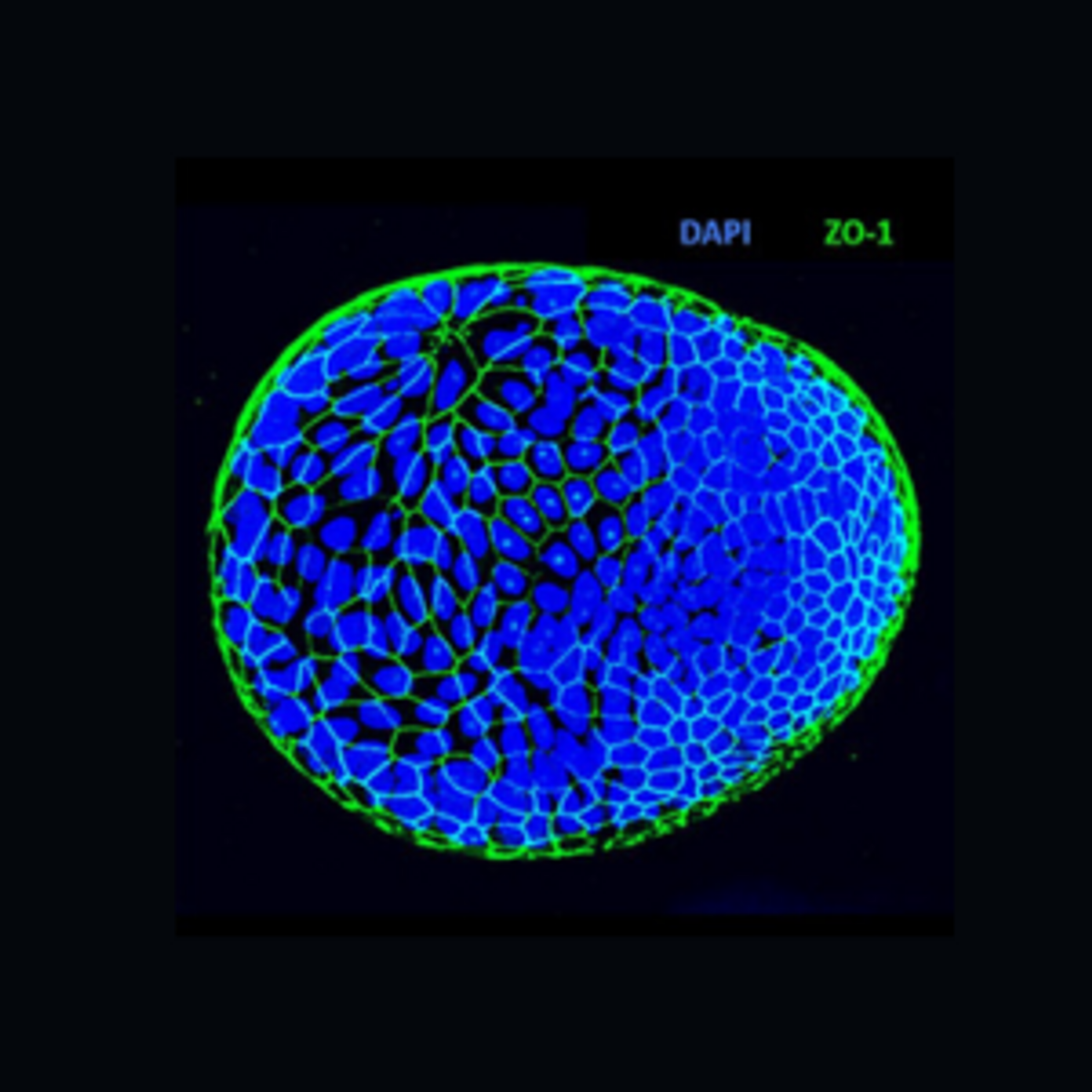
Recently, Julia Co and her colleagues published a paper that describes a rapid method to reverse the polarity of human gastrointestinal organoids.5 Through this protocol, researchers formed “apical-out” organoids, where the apical surface faces outwards towards the culture medium and is accessible for scientists to study epithelial barrier integrity, secretion, absorption, innate immune responses, and differentiation. To change organoid polarity, researchers first grow basolateral-out organoids and release them into suspension by depolymerizing the ECM proteins. As the organoids are no longer in contact with the ECM, their polarity becomes reversed. Scientists confirm this change to the apical-out morphology by immunostaining proteins with polarized distributions, such as labeling zonula occludens-1 for the apical tight junctions and β-catenin for the basolateral adherens junctions.
MilliporeSigma has recently presented researchers with multiple protocols and products to employ apical-out organoids in their laboratories. They modified the procedure published by Co and colleagues to produce apical-out gastric, ileum, and colon PDOs. To make PDOs more accessible to scientists, MilliporeSigma runs a gastrointestinal organoid biobank that stores 58 organoids generated from patient samples collected from various organs, such as the colon, duodenum, and stomach. Additionally, their biobank includes organoids derived from patients with cancer, ulcerative colitis, and irritable bowel syndrome. MilliporeSigma also supplies protocols for immunostaining or cryopreserving apical-out organoids, as well as methods to test their barrier integrity and susceptibility to Helicobacter pylori infection. To assist with these procedures, they offer a wide variety of products, including cell strainers, growth factors, ECM hydrogels, conditioned media, and antibodies. Apical-out organoids, like the ones generated through MilliporeSigma’s protocol, are more versatile than basolateral-out organoids and will help accelerate research of epithelial health and disease.
References
- Tang XY, et al. Human organoids in basic research and clinical applications. Signal Transduct Target Ther. 2022;7(1):168.
- Kim J, et al. Human organoids: Model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571-584.
- Tortorella I, et al. The role of physical cues in the development of stem cell-derived organoids. Eur Biophys J EBJ. 2022;51(2):105-117.
- Williamson IA, et al. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell Mol Gastroenterol Hepatol. 2018;6(3):301-319.
- Co JY, et al. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat Protoc. 2021;16(11):5171-5192.
3dGRO™ organoids were derived utilizing HUB Organoid Technology.
The Life Science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada.