Stay up to date on the latest science with Brush Up Summaries.
What Are Peripheral Blood Mononuclear Cells?
Human peripheral blood mononuclear cells (PBMCs) are immune cells in the peripheral blood that have a single, round nucleus. PBMCs include lymphocytes, monocytes, and their derivative cells. For research and clinical purposes, mononuclear cells are isolated from leukopaks, which are blood samples enriched by extracting white blood cells from the peripheral blood.1,2
How Do Researchers Isolate PBMCs?
What is leukapheresis?
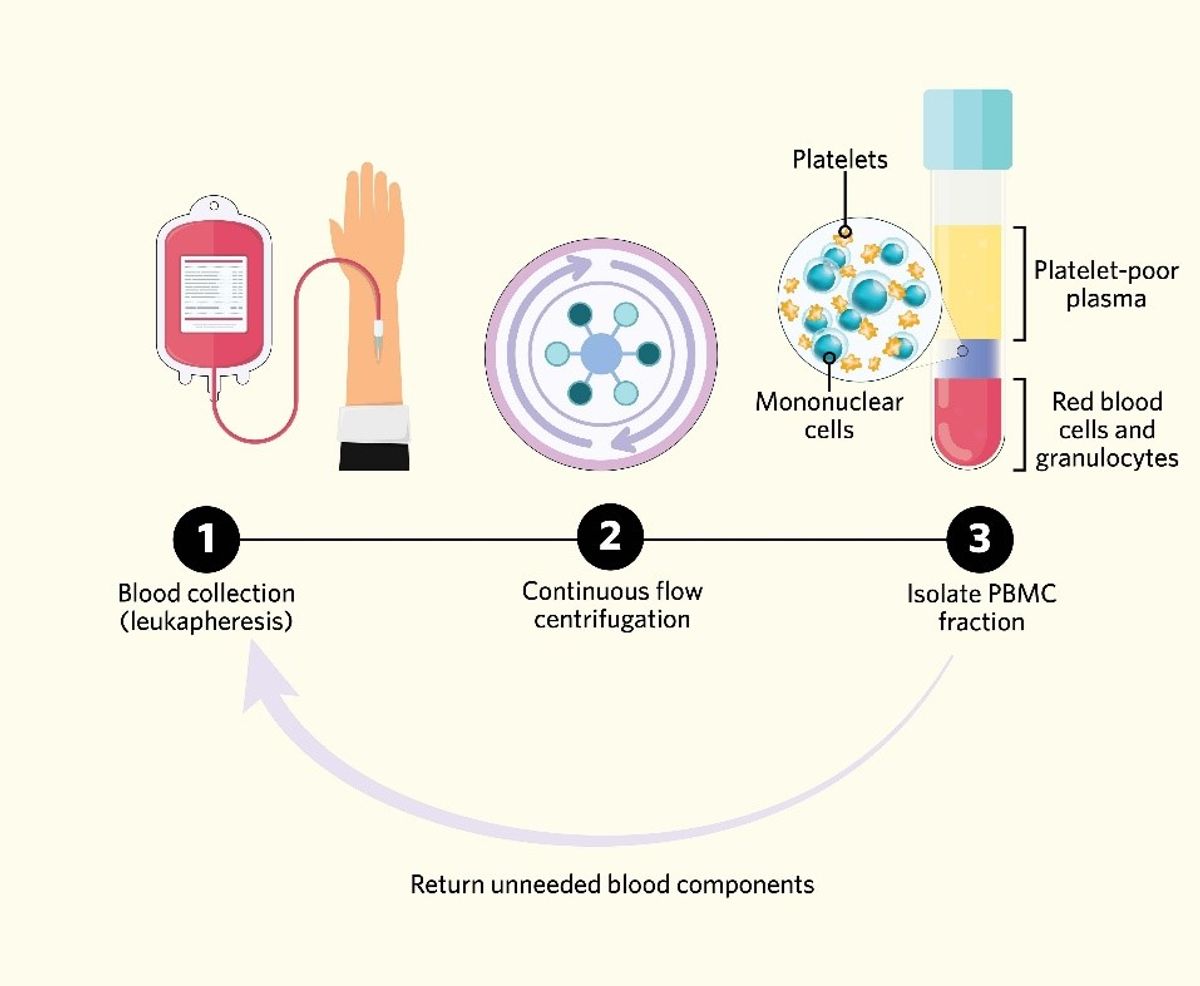
Scientists collect leukopaks from the peripheral blood of healthy human donors by leukapheresis—a procedure that separates blood components to collect specific cells and return the unneeded constituents back into circulation.1-3 Typically, health care professionals employ continuous flow centrifugation (CFC) to collect, spin, and return blood continuously while the donor is connected to an instrument called an apheresis circuit. CFC enriches and collects PBMCs by separating whole blood into fractions based on density. Beneath the plasma layer, PBMCs are located in the upper, low-density fraction produced by centrifugation, while red blood cells and granulocytes are in the lower, high-density fraction. After separation, the fractions that do not contain PBMCs are returned to the patient by the apheresis circuit.1,2,4 This system’s main advantage is that there is only a small volume of blood outside of the donor’s circulation during the procedure. Because of this, patients do not typically need fluid replacement during leukapheresis.2
Types of isolated mononuclear cells
PBMCs include lymphocytes such as T cells, B cells, and NK cells, and monocytes such as dendritic cells and macrophages. In humans, the frequencies of these cell types vary. Typically, lymphocytes account for roughly seventy to ninety percent of PBMCs and monocytes make up approximately ten to twenty percent. No matter the specific cell type, PBMCs are essential mediators of immune homeostasis and inflammation.1
Bisulfite conversion is the chemical reaction that occurs when scientists treat DNA with sodium bisulfite. In 1992, researchers found that the amination reaction of sodium bisulfite with unmethylated cytosine is different than the reaction of sodium bisulfite with 5mC. Because of this difference, unmethylated cytosines in single-stranded DNA become uracil residues after exposure to sodium bisulfite, while 5mCs remain cytosines. Pretreatment with sodium bisulfite is the basis of many methylation detection and analysis techniques.1 Following bisulfite conversion, researchers determine the methylation status in loci of interest because unmethylated cytosines are recognized as thymine after PCR amplification and sequencing.3
How Do Researchers Use PBMCs?
Immunotherapy and oncology
Researchers commonly use PMBCs to better understand immunological diseases and to develop immunotherapies. For instance, scientists study PBMCs to investigate the pathogenesis of asthma and other allergic diseases that involve various immunological pathways.5,6 Additionally, PBMCs are key to manufacturing chimeric antigen receptor T cell (CAR T) therapies for cancer treatment. Scientists isolate PBMCs from patients via leukapheresis at the beginning of the CAR T manufacturing process. Researchers reprogram the isolated T cells with gene editing, which allows the T cells to identify and eliminate cancer cells that express a specific target antigen. Several CAR T therapies are approved by the FDA for treatment of malignancies such as B cell lymphoma and multiple myeloma.7
Vaccine development and infectious disease
Many scientists employ and examine PBMCs in infectious disease research and vaccine development. Researchers glean information about the immune response through studying the frequency of specific PBMC subsets in circulation. This is useful for a host of applications, including understanding which cell types are involved in fighting different infections, examining the immune response to specific antigens, and determining immune activation after vaccination.8 For example, investigators have been studying PBMC-mediated responses to SARS-CoV-2 infection to understand how coordinated and focused immune responses contribute to successful viral elimination.9
Transplant and regenerative biology
Blood is the most convenient source of patient-derived stem cells from patients, and is a large, accessible source of adult stem cells for both basic research and clinical applications. PBMC populations contain many types of distinct multipotent progenitor cells that can differentiate into specific cell types under the right conditions, including blood cells, endothelial cells, hepatocytes, cardiomyogenic cells, smooth muscle cells, osteoblasts, osteoclasts, epithelial cells, neural cells, and myofibroblasts. Researchers can also expand PBMC populations in culture and reprogram them into induced pluripotent stem cells (iPSCs). Scientists transplant peripheral blood-derived stem cells to regenerate tissues and restore function after injury, making these cells ideal for clinical applications in regenerative medicine.10
References
- C.R Kleiveland, “Chapter 15 Peripheral Blood Mononuclear Cells,” in The Impact of Food Bioactives on Health: in vitro and ex vivo models, K. Verhoeckx et al., eds., Springer, 2015, p. 161-67.
- A. Garcia et al., “Leukopak PBMC sample processing for preparing quality control material to support proficiency testing programs,” J Immunol Methods, 409:99-106, 2014.
- NCI Dictionary, “Leukapheresis,” National Institutes of Health, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/leukapheresis, accessed November 29, 2022.
- M. Bharadwaj et al., “Detection and characterisation of alloreactive T cells,” Methods Mol Biol, 882:309-37, 2012.
- D. He et al., “Whole blood vs PBMC: compartmental differences in gene expression profiling exemplified in asthma,” Allergy Asthma Clin Immunol, 16:67, 2019.
- M.A. Pasha et al., “Role of innate lymphoid cells in allergic diseases,” Allergy Asthma Proc, 40(3):138-45, 2019.
- J. Abraham-Miranda et al., “CAR-T manufactured from frozen PBMC yield efficient function with prolonged in vitro production,” Front Immunol, 13:1007042, 2022.
- A.J. Pollard, E.M. Bijker, “A guide to vaccinology: from basic principles to new developments,” Nat Rev Immunol, 21(2):83-100, 2021.
- S.C. Jordan, “Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses,” Clin Exp Immunol, 204(3):310-20, 2021.
- M. Zhang, B. Huang, “The multi-differentiation potential of peripheral blood mononuclear cells,” Stem Cell Res Ther, 3(6):48, 2012.