ABOVE: Moungi Bawendi (left), Louis Brus (center), and Alexei Ekimov (right) won the 2023 Nobel Prize in Chemistry. Ill. Niklas Elmehed © Nobel Prize Outreach
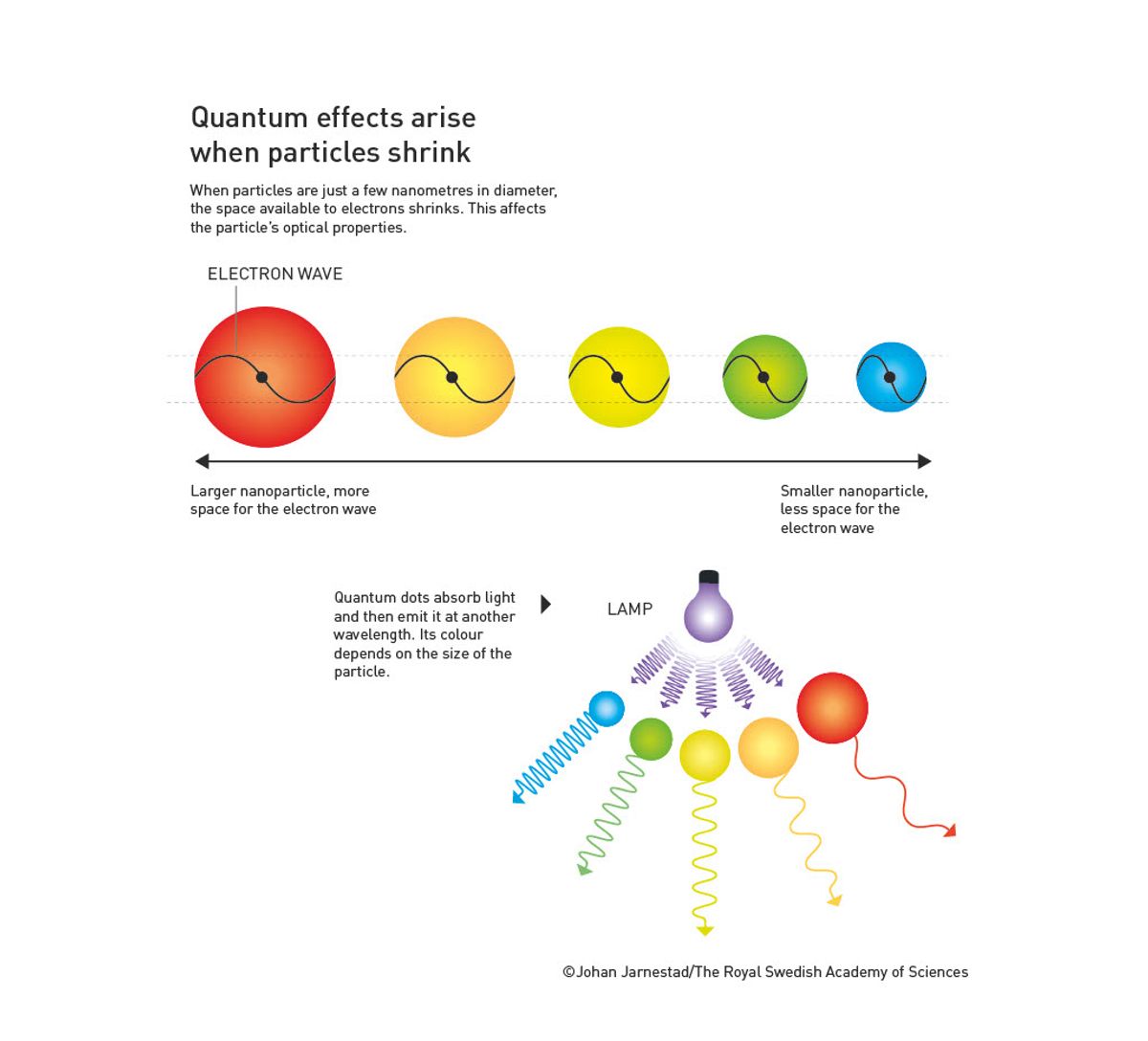
The 2023 Nobel Prize in Chemistry has been awarded to Moungi Bawendi of the Massachusetts Institute of Technology, Louis Brus of Columbia University, and Alexei Ekimov of Nanocrystals Technology Inc. “for the discovery and synthesis of quantum dots.” The properties of these nanoparticles such as color and thermal characteristics change with their size.
“These achievements represent an important milestone in nanotechnology. And today, there are numerous applications of quantum dots, ranging from QLED screens to imaging in biochemistry and medicine, and much more,” said Johan Åqvist, a biochemist at Uppsala University and chair of the Nobel Committee for Chemistry, at the press conference.
As early as the 1930s, researchers like physicist Herbert Fröhlich at the University of Bristol had predicted the unusual properties of nanoparticles, but for many years, researchers were unable to test them. “For the next five decades, it seemed nearly impossible to make such materials,” said Heiner Linke, a nanoscience researcher at Lund University and member of the Nobel Committee for Chemistry, at the press conference.
See also “Nobel Prize for mRNA Vaccines”
In 1981, however, Ekimov, working at the S. I. Vavilov State Optical Institute in the former Soviet Union, made a groundbreaking discovery by measuring the light absorption of colored glass.1 At the time, scientists knew that adding a single substance to glass could result in different colors of glass, depending on how the material was heated and cooled. Ekimov used copper chloride to color glass by heating the glass to different temperatures and cooling it for different lengths of time. This produced tiny copper chloride crystals of different sizes. When he measured the light absorption of the glass, he found that the size of the particles affected their light absorption; smaller particles absorbed bluer light. Ekimov realized that he had demonstrated a size-dependent quantum effect, and although he published his findings in a Soviet research journal, his research was difficult to access in the United States.
See also “Biocompatible Reactions In Living Cells Garner Chemistry Nobel”
Unaware of Ekimov’s research, Brus, working at Bell Laboratories in the United States at the time, independently produced quantum dots of his own two years later.2 Brus experimented with tiny particles of cadmium sulfide for his work on using solar energy to drive chemical reactions. He noticed that the optical properties of these particles changed over time, and hypothesized that it was because the particles were growing larger. He then compared particles of different sizes and observed the same size-dependent quantum effect, becoming the first person to discover quantum dots in solution.
“However,” said Åqvist, “for these quantum dots to become really useful, [scientists] needed to be able to make them in solution with exquisite control of their size and surface. Moungi Bawendi invented an ingenious chemical method for doing just this. He could now make perfect nanoparticles of very specific size and very high quality.” Bawendi’s precise synthesis technique, published in 1993, enabled the wider commercial use of quantum dots.3
While quantum dots are not yet widely used in biomedicine, they have many potential applications, including imaging of living cells and tumors, phototherapy for cancer, and traceable drug delivery.4 Quantum dots are still an active area of research and, said Linke, “we believe that the really big applications are still in the future.”
References
- Ekimov AI, Onushchenko AA. Quantum size effect in three-dimensional microscopic semiconductor crystals. Soviet Journal of Experimental and Theoretical Physics Letters. 1981;34:345.
- Rossetti R et al. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. The Journal of Chemical Physics. 1983;79(2):1086-1088.
- Murray CB et al. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115(19):8706-8715.
- Abdellatif AAH et al. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int J Nanomedicine. 2022;17:1951-1970.








