In solid cancers, cellular behaviors such as motility and invasiveness are well characterized contributors to poor prognosis and cancer spread. Scientists pay close attention to a process called epithelial to mesenchymal transition (EMT) in solid cancers, as this transformation primes malignant cells for metastasis. However, EMT is somewhat of an enigma for blood and lymphatic cancers, which originate from cells that already freely circulate in the body before becoming malignant. Although conventional EMT transcription factors are often differentially expressed in leukemias and lymphomas, their oncogenic role remains unclear in different hematopoietic contexts.1
Pathologist and researcher Charles Mullighan from St. Jude Children’s Hospital uses a variety of in vitro and in vivo models to investigate high risk leukemia pathogenesis. His research team’s latest work, published in Cell Reports, homed in on cellular interactions between acute lymphoblastic leukemia (ALL) and the bone marrow, and discovered downstream EMT pathways that enhance cancer cell survival.2
“We sought to perform a comprehensive analysis of the functional changes that occurred in leukemic cells on engagement with stroma and how that influenced drug responsiveness,” Mullighan said via email.
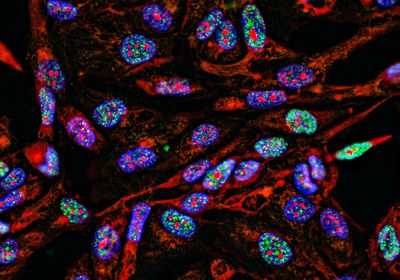
The researchers used an ex vivo co-culture model to investigate the interaction between ALL cells and mesenchymal stem cells (MSCs), a subset of bone marrow stem cells that promote ALL survival and drug resistance.3 Using CRISPR-based screens and RNA sequencing, Mullighan’s team uncovered the molecular underpinnings of EMT in leukemia cell lines and patient-derived ALL cells. “The profound transcriptional deregulation and activation of multiple cellular activation/signaling pathways, including EMT, and the downstream effector pathways of EMT, was unexpected,” wrote Mullighan. “Although multiple pathways downstream of EMT were activated, only some appeared to have a role in leukemic maintenance or drug sensitivity.” The scientists discovered that integrin β1-mediated ALL-MSC interactions induce an EMT-like state in leukemia cells primarily through WNT/β-catenin signaling, which caused drug resistance and enhanced cancer cell survival.
What makes a leukemia bad is more its capacity to stick somewhere. So, I would say that even just theoretically, this is exactly what an EMT—a tumor becoming bad—in leukemia would be.
-Diana Passaro, Cochin Institute, Université Paris Cité
The bone marrow microenvironment influences leukemia initiation, progression, and survival through cellular crosstalk between stromal cells, immune cells, adipocytes, and neural cells.4 Mullighan’s discovery that stromal cell adhesion in this niche promoted EMT may seem counterintuitive because EMT is typically linked cell motility in solid cancers, but these findings align with current hypotheses in the field. “For leukemia, it's slightly different because the tumor itself, it's not fixed somewhere. The cells that get the mutation and start becoming malignant … they move around, they circulate. It’s not really a method of being motile, but they do flow rapidly within the marrow tissue, and they exit the marrow tissue rapidly. They flow into the blood,” said Diana Passaro, a bioengineer at the Cochin Institute who studies leukemia niche dynamics and who was not involved in the study. “What makes a leukemia bad is more its capacity to stick somewhere. So, I would say that even just theoretically, this is exactly what an EMT—a tumor becoming bad—in leukemia would be.”
See also “The Basics of the Tumor Microenvironment”
For Mullighan, the next step is pharmacologically targeting the pathways the team identified as integral in leukemic maintenance and drug sensitivity. Understanding the mechanisms of EMT is a jumping off point for developing specific inhibitors and preclinical models of ALL and other high risk leukemias. “The concept of the discovery is really exciting,” Passaro said. “It's great that it starts being characterized in leukemia, as it is characterized in solid cancers.”
References
- Radhakrishnan K, et al. An "unexpected" role for EMT transcription factors in hematological development and malignancy. Front Immunol. 2023;14:1207360.
- Park CS, et al. Stromal-induced epithelial-mesenchymal transition induces targetable drug resistance in acute lymphoblastic leukemia. Cell Rep. 2023;42(7):112804.
- Fallati A, et al. Mesenchymal stromal cells (MSCs): An ally of B-cell acute lymphoblastic leukemia (B-ALL) cells in disease maintenance and progression within the bone marrow hematopoietic niche. Cancers (Basel). 2022;14(14):3303.
- Bessy T, et al. Bioengineering the bone marrow vascular niche. Front Cell Dev Biol. 2021;9:645496.