The SARS-CoV-2 virus that causes COVID-19 (red cell) is covered in spike proteins (blue), which are glycoproteins that allow the virus to gain access to human cells.
The SARS-CoV-2 virus that causes COVID-19 (red cell) is covered in spike proteins (blue), which are glycoproteins that allow the virus to gain access to human cells. Stay up to date on the latest science with Brush Up Summaries.
What Are Glycoproteins?
Glycoproteins are a large and diverse group of proteins to which one or more sugar molecules, known as oligosaccharides, have been attached through covalent bonding. More than 50 percent of proteins in eukaryotes are known to be glycoproteins, with some predictions being as high as 70 percent.1
The many varieties of glycoproteins differ from each other in several key ways, including the type of oligosaccharide that is attached, its length, whether it is branched or linear, and where on the protein the attachment occurs.
How Do Glycoproteins Form and Where Do They End Up?
Glycoproteins are formed through glycosylation, which is a complex and reversible enzymatic reaction that transpires across all domains of life.2 Glycosylation can occur as a type of post-translational modification (PTM), or it can happen co-translationally, as is the case with N-glycosylation.3 There are six known types of glycosylation, resulting in different types of glycoproteins (Table 1), and some glycoproteins bear multiple sites of glycosylation.
Table 1: Types of Glycosylation and Their Attachments
Type of glycosylation | Form and attachment |
Oligosaccharides are linked to the nitrogen atom on an asparagine residue4 | |
Oligosaccharides are linked to oxygen, typically on serine or threonine residues5 | |
Carbon-carbon bonds link mannose to the indole ring of tryptophan6 | |
Oligosaccharides are linked to the sulfur atom of cysteine residues7 | |
Phosphodiester bonds link glycans to serine or threonine residues8 | |
Glycosylphosphatidylinositol anchor is added (protein and phospholipid linked by a glycan core)9 |
N-glycosylation and O-glycosylation are the most common forms,1 while phosphoglycosylation and S-glycosylation are extremely rare. Depending on the type, glycosylation occurs in different parts of the cell, primarily the endoplasmic reticulum (ER) and the Golgi apparatus.10
The addition of carbohydrates to proteins via glycosylation affects how proteins fold, provides specific instructions on where they will be trafficked, and allows them to perform a wider range of functions.11 Glycoproteins make up the majority of soluble proteins because they are hydrophilic, and most membrane proteins are also glycoproteins.12 The oligosaccharide chains of membrane glycoproteins are always positioned on the outside of the lipid bilayer of the cell, coating eukaryotic cells with these carbohydrates. This coating is called the cell coat or glycocalyx.12
What Functions Do Glycoproteins Have?
Glycoproteins are incredibly diverse and have myriad functions within organisms, including roles in development, growth, homeostasis, and survival.13 They are crucial for cellular interactions; secreted glycoproteins can act as signaling molecules and membrane-bound glycoproteins can function as the surface receptors to which those signaling molecules bind. A key example of this is glycoprotein hormones and their receptors, which are involved in human reproduction.14
Glycoproteins also function extensively in the human innate and adaptive immune system—in fact, almost all immune molecules are glycoproteins.15 For example, glycoproteins form the T cell receptor complex, the antibodies produced by B cells, and the major histocompatibility complex. Cytokines secreted by immune cells that control inflammation are also glycoproteins.16
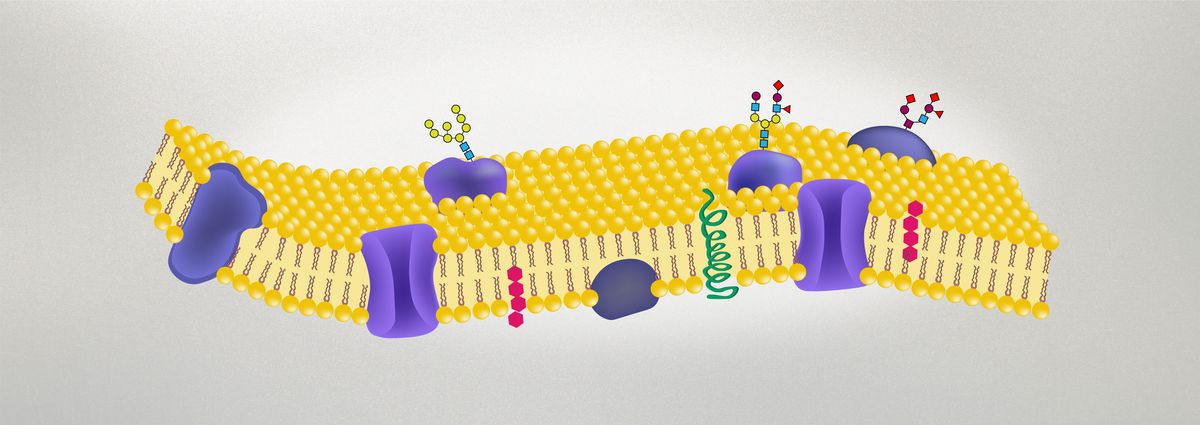
The Role of Glycoproteins and Aberrant Glycosylation in Disease
As glycoproteins are involved in many critical physiological processes, aberrant glycosylation can have significant negative consequences to human health.5 As such, scientists have explored many glycoproteins as therapeutic targets. A recombinant version of erythropoietin, the glycoprotein hormone responsible for stimulating the production of red blood cells, is used to treat patients with anemia.17
Because of their various roles in disease, as well as their accessibility in bodily fluids, scientists have used many secreted glycoproteins as disease biomarkers.18,19 Inflammatory cytokines, for example, are involved in the pathogenesis and are robust predictors of a range of cardiovascular diseases. Overexpression of certain inflammatory cytokines is also responsible for the onset and progression of tumors,16 with researchers recognizing aberrant glycosylation as a key hallmark of cancer.
Glycoproteins also serve important functions in infectious disease, with glycoprotein receptors expressed on viral capsids involved in both recognition and infection of viable host cells.20 The SARS-CoV-2 virus, which causes COVID-19, gains access to human cells with its spike protein, which is a glycoprotein.21 Further studies of glycoproteins in humans and microorganisms will expand the understanding of disease pathogenesis and yield a greater range of disease biomarkers and therapeutic targets.

References
- An HJ et al. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol.2009;13(4):421-426.
2. Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R-56R.
3. Shrimal S et al. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin Cell Dev Biol. 2015;41:71-78.
4. Breitling J, Aebi M. N-linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(8):a013359.
5. Reily C et al. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346-366.
6. Furmanek A, Hofsteenge J. Protein C-mannosylation: facts and questions. Acta Biochim Pol. 2000;47(3):781-789.
7. Stepper J et al. Cysteine S-glycosylation, a new post-translational modification found in glycopeptide bacteriocins. FEBS Lett. 2011;585(4):645-650.
8. Boutin S et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704-712.
9. Roller RF et al. Semisynthesis of functional glycosylphosphatidylinositol-anchored proteins. Angew Chem Int Ed Engl. 2020;59(29):12035-12040.
10. Ramazi S, Zahiri J. Post-translational modifications in proteins: resources, tools and prediction methods. Database. 2021;2021:baab012.
11. Jayaprakash NG, Surolia A. Role of glycosylation in nucleating protein folding and stability. Biochem J. 2017;474(14):2333-2347.
12. Alberts B et al. Molecular Biology of the Cell. 5th ed. (Anderson M, Granum S, eds.). Garland Science; 2008.
13. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97-130. doi:10.1093/glycob/3.2.97
14. Szkudlinski MW. New frontier in glycoprotein hormones and their receptors structure–function. Front Endocrinol. 2015;6. https://www.frontiersin.org/articles/10.3389/fendo.2015.00155
15. Rudd PM et al. Glycosylation and the immune system. Science. 2001;291(5512):2370-2376.
16. Amin MN et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020;8.
17. Ng T et al. Recombinant erythropoietin in clinical practice. Postgrad Med J. 2003;79(933):367-376.
18. Suttapitugsakul S et al. Enhancing comprehensive analysis of secreted glycoproteins from cultured cells without serum starvation. Anal Chem. 2021;93(4):2694-2705.
19. Chandler K, Goldman R. Glycoprotein disease markers and single protein-omics. Mol Cell Proteomics. 2013;12(4):836-845.
20. Garcia NK, Lee KK. Dynamic viral glycoprotein machines: approaches for probing transient states that drive membrane fusion. Viruses. 2016;8(1).
21. Duan L et al. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: implications for the design of spike-based vaccine immunogens. Front Immunol. 2020;11.