ABOVE: modified from © istock.com, elena
We first met Kate and her husband Adam in 2016 when she was 26 weeks pregnant and in labor. Within hours, she gave birth to twins, James and Fraser. The newborns weighed in at just around 1 kilogram each. “Seeing them—so tiny and fragile, but alive—flooded my body with sheer relief,” Kate wrote of the experience in an email. “I touched their little hands before they were wheeled away into the Neonatal Intensive Care Unit (NICU).”
Kate pumped breast milk that was fed to the newborns to help them grow. But before long, James took a turn for the worse, developing a devastating intestinal condition known as necrotizing enterocolitis. Surgeons needed to cut a tiny hole in his belly to allow gas to be released from his bowel, but bacteria from his damaged colon had spread through his body. After just three and a half weeks, we had to tell Kate and Adam that James wouldn’t survive, and that it was time to say goodbye.
Unfortunately, James’s story is not unique. Millions of little lives are lost around the time of birth every year. Some babies don’t make it as long as James did, dying even before delivery. Every 16 seconds a baby is stillborn somewhere in the world; this amounts to more than 2 million stillborn babies globally every year. Of babies that are born alive, shockingly high numbers of them are born too early. A baby is born prematurely every two seconds, resulting in 15 million preterm babies every year. Sadly, 1 million of these preterm babies die every year due to direct complications from preterm birth, and another 800,000 of them die from infections associated with preterm birth. Preterm birth remains the leading cause of death for babies and young children the world over.
Adverse pregnancy outcomes and the associated fetal, neonatal, and maternal deaths constitute the longest, deadliest pandemic of human history.
See “Why So Soon?”
Preterm birth is also the leading cause of childhood disability, with 1.3 million preemies every year suffering major disabilities such as breathing difficulties, blindness, and cerebral palsy. Moreover, susceptibility in the perinatal window is not limited to babies. Nearly 300,000 mothers die every year due to complications of pregnancy and childbirth. Together, adverse pregnancy outcomes and the associated deaths and disabilities constitute the deadliest and longest pandemic of human history.
Despite the high and steady death toll, vulnerability during pregnancy and early in life has for too long been accepted as unavoidable. Although adverse pregnancy outcomes consistently rate as one of the top three causes of death across the entire human lifespan, research to address it receives less than 1 percent of total funding. This disconnect has been thrown into stark relief by the ongoing COVID-19 pandemic. Not only does SARS-CoV-2 represent yet another pathogen that increases risk for stillbirth, preterm birth, and maternal death, uncertainty surrounding the risks to pregnant people and their fetuses who are exposed to the virus highlights the broader reality that the immunology of pregnancy remains largely enigmatic and understudied.
See “How COVID-19 Affects Pregnancy”
Indeed, much remains unknown about the factors associated with adverse pregnancy outcomes. Nevertheless, the little that is known strongly supports the idea that modulation of the mother’s immune system—for example, through diet or maternal vaccination—can improve pregnancy outcomes. Furthermore, given the scalability of these interventions, finally addressing this massive crisis is within reach.
For the duration of pregnancy, immune tolerance of the baby, who is genetically foreign to the mother’s body, is critical. So is immune resilience—avoiding undue inflammation, for example, in the face of benign commensal microbes. But inflammation is required for the separation of the maternal and fetal layers of the placenta that occurs leading up to birth. The timing and induction of this inflammation is tightly controlled by physiological signals from both the fetus and the mother around 37 to 42 gestational weeks. Anything that activates this inflammatory cascade too early—what’s known as aberrant immune activation—can result in the premature separation of the maternal and fetal placental layers, ultimately causing preterm birth. Interventions that avert unnecessary inflammation thus should reduce the risk of pregnancy complications. By applying the tools of modern science to understand the immunological dynamics of gestation, we may be able to save millions of lives and put an end to this substantial cause of suffering.
Immunobiology of pregnancy
Pregnancy is an immunological marvel, representing the only natural physiological state where genetically foreign cells and tissues lie in close physical contact with the host immune system without rejection. What prevents maternal immune cells from attacking fetal tissues remains unclear. Aberrant activation of maternal immune components associated with pregnancy complications such as prematurity likely represents defects in pregnancy-induced immune tolerance and resilience.
Interestingly, prior pregnancies appear to protect against such complications in future pregnancies. Mothers of sons immunologically remember their babies thanks to long-lived T cells with specificity for Y-chromosome-encoded antigens. Recent characterizations of these fetal-specific T cells in mice have revealed that pregnancy stimulates maternal T cells to adopt functionally unique properties. Researchers have shown, for example, that maternal CD8+ killer T cells develop an exhaustion-prone phenotype, meaning that they selectively silence killer-cell properties upon re-encountering fetal antigens in subsequent pregnancies. At the same time, one of us (S.S.W.) and colleagues have shown that pregnancy stimulates the differentiation of CD4+ T cells, which are dedicated to suppressing, instead of activating, other immune cells. Persistence of these immune-suppressive T cells after pregnancy may explain why the incidence of pregnancy complications is sharply reduced in second compared with first pregnancies—and why these protective benefits appear to be paternity-specific. Such immune tolerance may be further enforced by fetal cells that continue to circulate in the mother’s bloodstream.
Deploying vaccines not only to prevent infection with specific pathogens, but for their immune-modulatory potential, could save millions of lives.
A mother cannot afford to totally suppress her immune system, however, as pathogens are an ever-present threat. In addition to warding off infection as well as possible during pregnancy, a mother’s body will send immune sentinels across the placenta to provide protection to the baby after it’s born. For example, we and others have found that transfer of maternal antibodies to the fetus occurs in utero, ramping up significantly at 30–34 weeks gestation. Transfer of immunological experience continues postnatally through breast milk, which provides protective benefits to babies beyond the neonatal window.
Although such vertical transmission of antibodies has long been recognized, details of such immune sharing continue to be unveiled. Earlier this year, one of us (S.S.W.) and colleagues found that pregnancy actively modifies the molecular structure of antibodies, expanding their protective scope beyond extracellular pathogens to include immunity against microbes that live inside cells. This resolves a long-standing conundrum for how antibodies work against pathogens such as HIV, tuberculosis, or Zika virus that live inside cells and thus were once thought to be hidden from antibodies. It also implies that maternal antibodies are not simply immunological effectors, but also serve to activate and regulate an infant’s developing immune system, supporting the idea that vaccinating expecting mothers or reproductive-age women (and other individuals capable of pregnancy) prior to conception helps young babies in developing their own defense against microbes.
Of course, not all microbes that we encounter are pathogenic; many are harmless or even beneficial. In the context of pregnancy, controlling inflammation induced by microbes in the birth canal is likely important, as vaginal dysbiosis has increasingly been linked with prematurity and other pregnancy complications. For example, spontaneous preterm birth is consistently linked with depletion of Lactobacillus crispatus species and high diversity of other vaginal microbiota. From the babies’ perspective, recognizing the difference between microbial friend and foe is critical at birth, as they undergo an abrupt transition to the external world and its plethora of commensal microbes. In this context, microbe-induced inflammation is likely to be more damaging than helpful. Here again, immune molecules, including antibodies, transferred from the mother to the newborn are likely key for the regulation of baby’s response to unharmful microbes.
The intricacy of immune regulation in pregnancy remains largely a black box, with many fundamental questions left unanswered. Answering such questions will be essential to understanding and addressing the aberrant maternal immune activation that can lead to adverse pregnancy outcomes.
Immunology During PregnancyDuring pregnancy, the immune system adapts to support the baby’s development and coordinate birth. When immunity goes awry, so can the pregnancy, with adverse outcomes such as preterm birth and stillbirth often resulting from aberrant immune activation. Diet or maternal vaccination are examples of how to modulate the immune system to improve pregnancy outcomes. 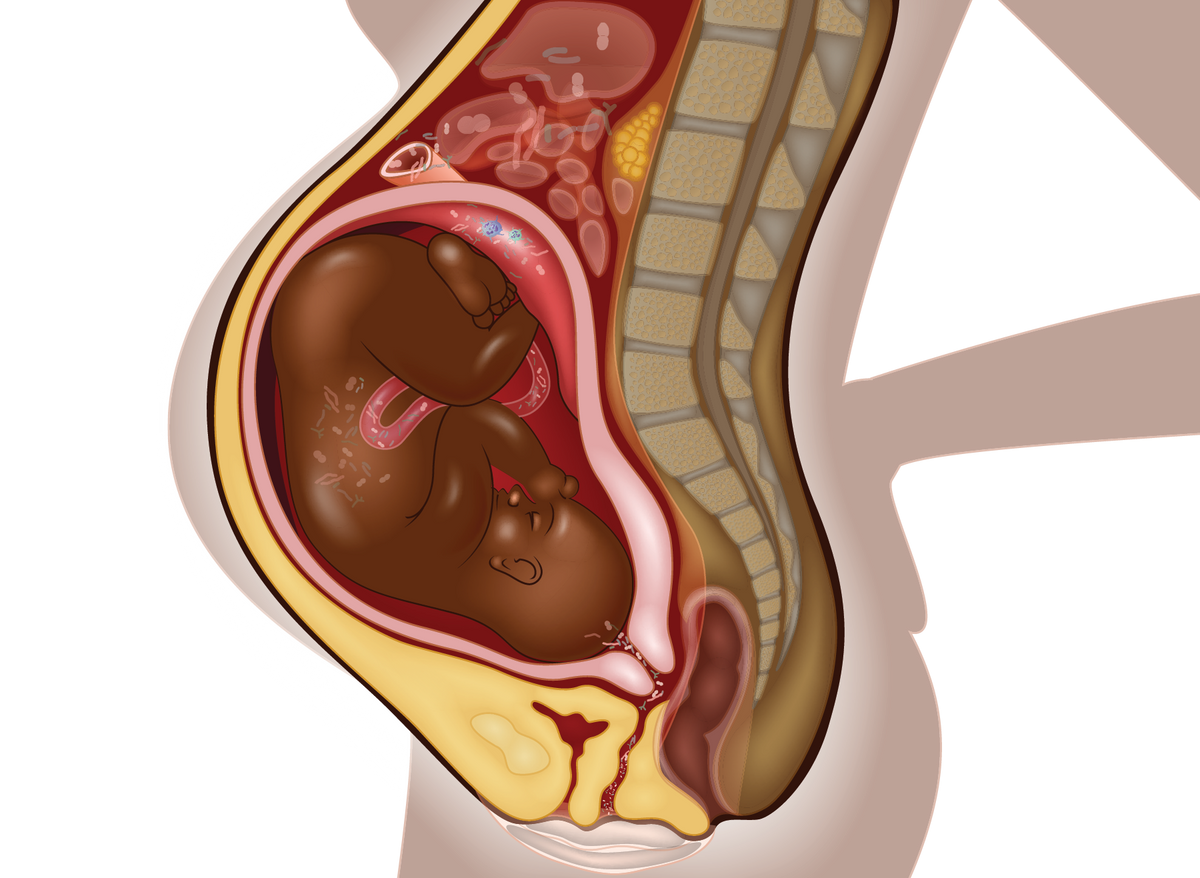 | |
IMMUNE TOLERANCE AND RESILIENCEPregnant individuals must both tolerate a genetically foreign fetus (immune tolerance) and avoid overreacting to the presence of microbes with inflammatory cascades that could jeopardize the pregnancy (immune resilience). To do this, they generate exhaustion-prone T cells that selectively silence killer-cell properties as well as long-lived immunosuppressive T cells. Both appear critical to a healthy pregnancy by averting aberrant immune activation. Conversely, an imbalance in the commensal microbes of the birth canal can trigger immune responses that have been linked with prematurity and other pregnancy complications.  | |
VERTICAL TRANSFER OF MATERNAL IMMUNITYAntibodies and other immune factors can pass across the placenta from mother to child, as well as through breast milk after birth. This means that a mother’s acquired immunity to pathogens, including through vaccination, can protect the baby after birth. In addition to providing postnatal protection against specific pathogens, maternal immune molecules transferred to the baby can regulate the fetal and newborn immune system. Such factors can support the baby’s in utero immune tolerance to the genetically foreign mother as well as its immune resilience before and after birth, avoiding excessive immune activation by commensal microbes. |  |
INFLAMMATORY CASCADE CONTROLS BIRTH TIMINGWhile aberrant immune activation can be disastrous, inflammation plays an important role in the process of birth. Typically initiated starting around 37 to 42 gestational weeks, these inflammatory signals can be prematurely activated and trigger the separation of the maternal and fetal placental layers, leading to preterm birth or stillbirth. Certain dietary interventions such as supplements of omega-3 fatty acids or the amino acid L-arginine have been shown to protect against preterm labor in some populations, and may act by reducing inflammatory processes. | 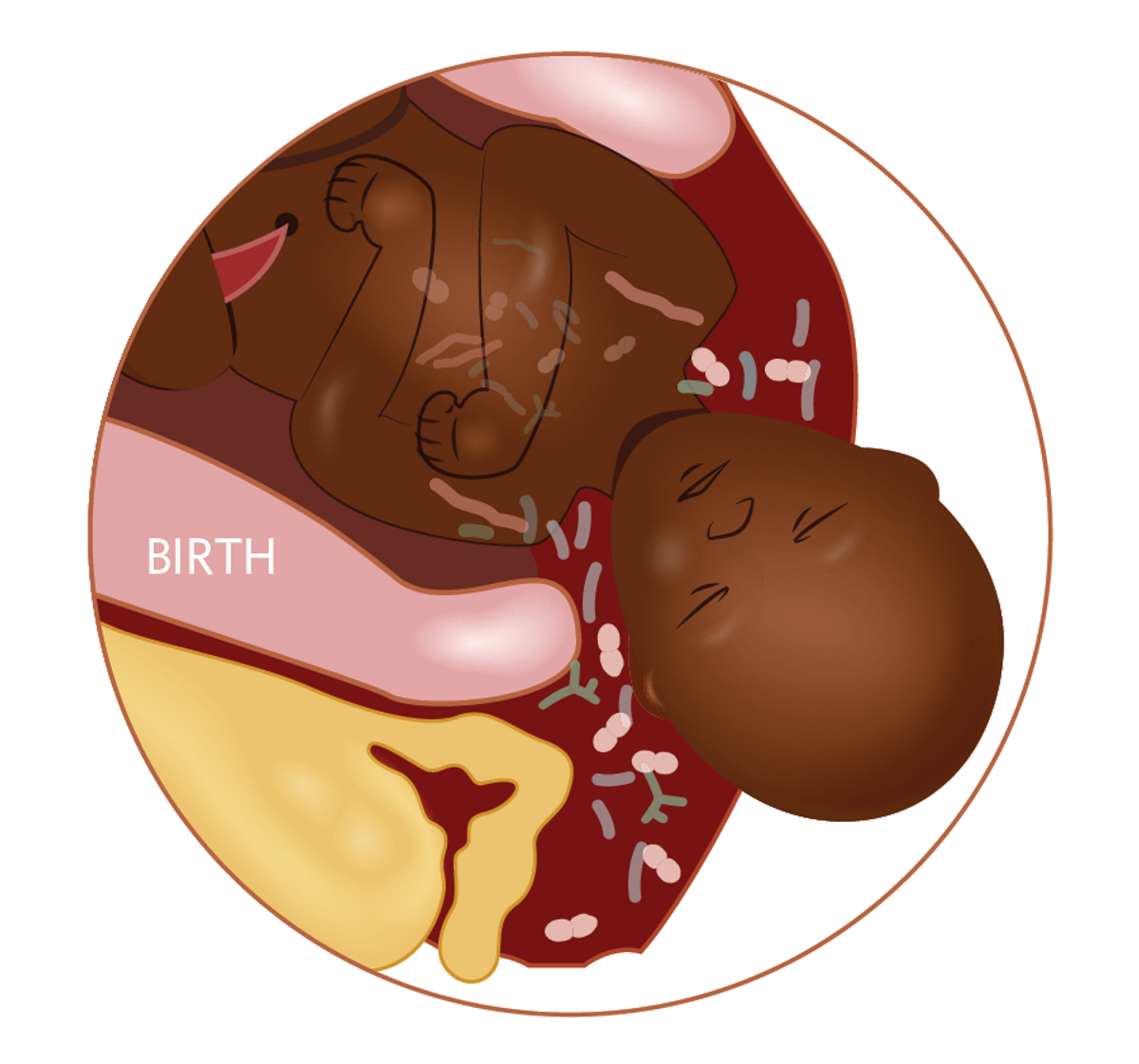 |
Immunological interventions to improve pregnancy outcomes
Even with the current, relatively rudimentary understanding of the immunological processes at play during pregnancy, there are immune-modulatory approaches clinicians are already employing to protect pregnancies and newborns. The most direct approach relates to maternal vaccination against common diseases. As infections are generally associated with adverse pregnancy outcomes, protecting mothers against infection can help reduce the risk of preterm births and stillbirths. Such protective effects have been noted with maternal influenza and pertussis immunization already, where reduction of risk for stillbirth or preterm birth was as high as 50 percent in mothers who received either influenza or pertussis vaccinations, or both. When it comes to COVID-19, data point to a higher risk of preterm birth and stillbirth following maternal infection, indicating that maternal vaccination not only protects the mother from severe disease but can also prevent adverse pregnancy outcomes.
Maternal vaccination against various pathogens has also long been recognized for its ability to protect newborn babies, thanks to the antibodies transferred in utero and in breast milk. For example, maternal tetanus vaccination, together with improved birth and umbilical cord hygiene practices, has reduced neonatal mortality from tetanus by nearly 90 percent over the last decade. Similarly, maternal pertussis vaccination prevents severe whooping cough early in the baby’s life.
Importantly, vaccines modulate the immune system in ways far beyond pathogen-specific immune responses as well. This has been well documented in non-pregnant vaccine recipients but is likely also occurring in women vaccinated during pregnancy. This suggests that maternal vaccination could be deployed to intentionally modulate the immune system of pregnant women to reduce aberrant immune activation and thereby protect against adverse pregnancy outcomes. For example, mothers experienced improved pregnancy outcomes following maternal influenza vaccination even outside of flu season, indicating pathogen-agnostic rather than only pathogen-specific benefits following vaccination during pregnancy. Additionally, a recent study demonstrated that BCG immunization of women prior to pregnancy also reduced the incidence of adverse pregnancy outcomes, suggesting vaccine-induced modulation of immune trajectories impacting pregnancy may extend to before the gravid period.
Given that the upstream causes of adverse pregnancy outcomes are not sufficiently well understood, it comes as no surprise that insights into vaccine-induced pathogen-agnostic immune-modulatory effects of maternal vaccination improving pregnancy outcomes are limited. Mechanisms could relate to overall immune regulation, such as increased resilience to various immune perturbations. They also could relate to increased innate immunity providing broad pathogen-agnostic protection from a variety of potential microbial culprits, akin to the concept of trained immunity.
See “How Some Vaccines Protect Against More than Their Targets”
Even less is known about how more-indirect immune-modulatory approaches such as dietary interventions reduce adverse pregnancy outcomes. For example, omega-3 fatty acids supplements, which have been shown to protect against preterm labor in populations where omega-3 deficiency is common, may act via systemic immune modulators of fatty acid origin (eicosanoids). Another supplement, the amino acid L-arginine, similarly appears to protect against preterm birth, as well as stillbirth, especially in malaria-infected women, possibly due to a reduction of inflammatory processes in the placental vascular bed.
Irrespective of the missing insight, data showing improved pregnancy outcomes for vaccinated mothers or those taking omega-3 or L-arginine supplements are proof that the global burden of pregnancy complications might be reduced through targeted interventions. Moreover, such interventions offer ideal opportunities to decipher underlying protective mechanisms. Our hope is that, in the future, maternal immunization and nutritional strategies can be improved and better integrated to optimally protect both mother and child.
Call to action
The scale of human suffering during the early life developmental window from conception to birth and beyond constitutes an ongoing public health emergency of frightening proportions. Tackling this problem also constitutes a massive opportunity. Specifically, implementing interventions of promise to provide rapid reprieve, along with deciphering how these interventions work, would establish the framework to safeguard all women and babies.
Groups around the world, such as the Born Strong Initiative, where one of us (T.R.K.) is chief executive officer, are actively pursuing this mechanistic understanding. The Born Strong Initiative’s studies on adverse pregnancy outcomes across the globe focus on interventions that are scalable and feasible for deployment in disadvantaged populations. The initiative’s basic approach of contrasting intervention arms with standard-of-care controls aims to reveal the missing mechanistic insight regarding how available interventions can most effectively prevent adverse pregnancy outcomes.
For these interventions to reduce the global incidence of adverse pregnancy outcomes, access and scalability are key. As stated in the World Health Organization’s pioneering Every Newborn Action Plan, “High-quality universal maternal and newborn care is not a privilege but the right of every child and every pregnant woman everywhere.” However, lack of access to quality healthcare is estimated to cause two-thirds of neonatal deaths and half of maternal deaths worldwide, with adverse pregnancy outcomes disproportionately affecting disadvantaged and marginalized populations.
Even among privileged factions of society, however, adverse pregnancy outcomes shockingly attract the lowest level of investment along the continuum of care. Compared with COVID-19, which received such a windfall of money and energy that effective vaccines were developed and made available within a year’s time, the resources available to scientists interested in understanding and improving pregnancy outcomes seems paltry, despite a death toll that has long outpaced that of the new coronavirus. In addition, there is a disconnect between maternal and newborn healthcare, each involving its own specialists with their own priorities. Yet, models of integrated mother-and-child care are now emerging, and the benefits are becoming apparent. For example, the management of maternal HIV infection and the prevention of newborn infection have led healthcare providers across the globe to join efforts in a multidisciplinary approach. This approach revealed the importance of effective control of maternal HIV infection for the health of the child, beyond the prevention of mother-to-child transmission of the virus. An integrated approach is clearly needed to optimize immune-modulatory interventions aimed at reducing stillbirth and preterm birth.
Although Kate and Adam’s son James was tragically lost, his twin brother Fraser eventually graduated from his 123-day stay in the NICU and is now “a typical six-year-old boy in every way,” Kate wrote in her note to us. Given his perseverance, his parents gave Fraser the nickname “The Beast.” In the future, we are hopeful that fewer babies born too early will have to fight as hard as Fraser did, the onus shifting more to the medical providers overseeing their mothers’ pregnancies, with better immunological control converting millions of missed opportunities into more healthy lives lived to their fullest potential.
Tobias R. Kollmann is a pediatric infectious disease clinician at Perth Children’s Hospital, director of Systems Immunology for the Human Vaccines Project, and chief executive officer of the Born Strong Initiative, a partnership between the Human Vaccines Project and Telethon Kids Institute aimed at reducing adverse pregnancy outcomes. Arnaud Marchant is codirector of the European Plotkin Institute for Vaccinology and director of the Institute for Medical Immunology of the Université libre de Bruxelles, Belgium. Sing Sing Way is a pediatric infectious disease clinician at Cincinnati Children’s Hospital, as well as director of the Center for Inflammation and Tolerance and the March of Dimes Ohio Collaborative on Preterm Birth.