ABOVE: Erkin Kuru (left), a synthetic biologist at Harvard University, and Helena de Puig (right), a biomedical engineer at the Wyss Institute, joined up to develop novel biosensors for rapid diagnostics. Max Rousseau; Tony Pulsone
Harvard University postdoctoral researcher Erkin Kuru’s journey in biological sciences started back in his undergraduate days in his home country of Turkey. Eventually, he decided to pursue a PhD in biochemistry at Indiana University in the lab of Michael VanNieuwenhze, where his research served as a bridge between biology and chemistry. “I’ve been fascinated by the synthetic biology field where we could engineer living systems to do our bidding,” said Kuru.
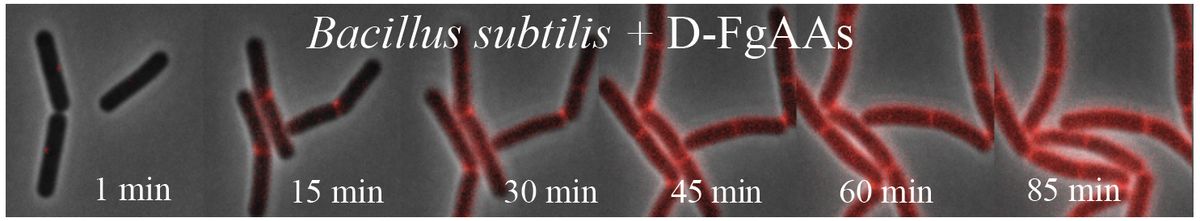
When Kuru joined the lab, VanNieuwenhze was looking for ways to label bacterial cell walls to track cell growth. Intrigued by the challenge, Kuru joined the effort. The researchers tried a few things, but nothing came to fruition. Finally, Kuru came across D-amino acids.
I’ve been fascinated by the synthetic biology field where we could engineer living systems to do our bidding.
—Erkin Kuru, Harvard University
The role of D-amino acids in biological processes is still an area of active research, but previous studies revealed that bacteria readily produce and incorporate them from the environment to modify cell walls.1 Innovating on this work, Kuru designed D-amino acid probes to fluoresce upon incorporation into key cell wall components, giving microbiologists a means to instantly track and visualize cell wall synthesis without perturbing the system.2-4
“Through persistence, through following up on his curiosity, [Kuru] landed upon fluorescent D-amino acids as a means to visualize bacterial cell wall growth and dynamics,” said VanNieuwenhze. “At the time, I did not understand how significant this discovery was.”
Following graduate school, Kuru joined the lab of George Church at Harvard Medical School with his sights set on refining his fluorogenic amino acid (fgAA) probes to develop faster and smaller protein labeling tools. Right as Kuru and his colleagues were strategizing ways to encode the fluorescent amino acids into proteins and subsequent assays, the COVID-19 pandemic hit. Kuru recalled lying in bed one night wondering what he could do to help the situation when he realized the answer: instant diagnostics.
Engineering rapid diagnostics
Around this time, Kuru met Helena de Puig, a postdoctoral researcher at the Wyss Institute who currently works in the lab of James Collins. After studying industrial engineering in her home country of Spain, de Puig went to the Massachusetts Institute for Technology (MIT) to dive into biotechnology. “I discovered at the beginning of my PhD that mechanical engineers could do something for healthcare,” said de Puig. While a graduate student in the lab of Lee Gehrke at MIT, de Puig developed rapid detection methods for diagnosing infectious diseases such as Ebola virus disease, Zika, and dengue fever.5,6
de Puig’s engineering background was critical for the design of the tests. “She did a huge amount of work on other aspects of developing lateral flow diagnostics that people sometimes don’t know about.” This included dealing with cross reactivity and background, which make it difficult to accurately detect a single virus.
I discovered at the beginning of my PhD that mechanical engineers could do something for healthcare.
—Helena de Puig, Wyss Institute
de Puig also brought with her valuable experience engineering gold nanoparticles.6 In lateral flow tests, gold nanoparticles shuttle specific targets in a sample towards the detection antibodies further down the membrane. “Her expertise allowed us to make not only gold nanoparticles, but a whole rainbow of different colors,” said Gehrke. “The reason that’s important is that you can use them for multiplexing and that’s huge for the diagnostics field.”
To gain more experience with synthetic biology techniques with the idea of eventually incorporating them into low cost diagnostics, de Puig joined the Collins lab where she worked with CRISPR-based diagnostics when the pandemic started.7 “I started working on COVID projects and I was put in contact with Erkin, who was working on the Sparkle projects, and they had a really cool diagnostic application for SARS-CoV-2,” said de Puig.
Kuru and de Puig share more than a common interest in rapid diagnostics; they approach science with the same fearless fervor. “[de Puig] is not afraid of trying new things and making new approaches,” said Gehrke. “She’s a big picture thinker and is always thinking broadly about the science and engineering of the tests but also how they can be applied to have the biggest effect on the largest number of people.”
Echoing this sentiment, VanNieuwenhze said, “[Kuru] is not afraid to try anything. He has a wealth of ideas and information, and he is always thinking in multiple directions at once.”
Shaking up science with sparkling sensors
Quick, affordable, and accurate are all characteristics of good diagnostics. But when Kuru and de Puig stepped back and looked at the bigger picture—how we identify biomolecules in samples—they noted several limitations. For example, immunohistochemistry relies on lengthy staining protocols with repeated wash cycles. Also, although a powerful tool, antibodies are not always sensitive or specific. Similarly, PCR-based diagnostics are time-consuming and require technical expertise. Instead of developing innovative solutions to any one of these problems, Kuru, de Puig, and their colleagues hope to revolutionize the entire process.
Sparkle is a Wyss Institute backed startup that aims to provide low-cost instant biosensors for a variety of applications. In addition to Kuru and de Puig, the technology development team includes Collins, Church, and Issac Han.
During his time in the Church lab, Kuru has been working to modify fgAAs and develop a generalizable platform for their development. The idea was to develop an assay with the accuracy of immunostaining but with an instant readout.
To do this, the researchers attached an fgAA to a nanobody designed to recognize and bind to a specific protein. This highly specific nanobody with its fgAA tentacle swims around in the dark, searching for its target. Almost instantly upon binding, the fgAA tentacle gets wedged between the nanobody and the target, locking it into a conformational position that leads to fluorescence. “It’s the simple idea that once it attaches, it shines,” said de Puig.
To put their “sparkle” sensors to the test, Kuru, de Puig, and their team designed a fluorogenic amino acid targeted to the Spike protein antigen of SARS-CoV-2. As promised, within half a second of a sample positive for COVID-19 encountering the biosensor, it sparkled.
These instant sparkly sensors radically reduce assay times from days down to mere milliseconds, making the technology particularly ideal for point-of-care settings. The team has several applications in mind. de Puig spoke of one example related to breast cancer. Following surgical resection, doctors send the tumor to pathology to check the tumor margins and confirm complete resection. “A crazy number of patients have to go back for a second surgery because the margins are positive for tumors,” said de Puig. A sparkle-based sensor targeting cancer cells could allow doctors to instantly check tumor margins while still in surgery where time is of the essence.
The team hopes to debut their patent-pending sparkle sensor platform later this year and expand beyond diagnostics towards therapeutic approaches. “Our platform will help us augment proteins with anything that we can imagine, and we are excited about that,” said Kuru. Adding to this, de Puig said, “My favorite part of this technology is that because you see things as our sensors bind to them, we can measure things in real time. This is something that we are bringing to the table that not a lot of other technologies can do.”
References
- Lam H, et al. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325(5947):1552-1555.
- Kuru E, et al. Synthesis of fluorescent D-amino acids and their use for probing peptidoglycan synthesis and bacterial growth in situ. Nat Protoc. 2015;10:33-52.
- Kuru E, et al. In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew Chem Int Ed Engl. 2012;51:12519-12523.
- Hsu Y, et al. Fluorogenic D-amino acids enable real-time monitoring of peptidoglycan biosynthesis and high-throughput transpeptidation assays. Nat Chem. 2019;11:335-341.
- de Puig H, et al. Multiplexed rapid antigen tests developed using multicolored nanoparticles and cross-reactive antibody pairs: Implications for pandemic preparedness. Nano Today. 2022;47:101669.
- Bosch I, et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci Transl Med. 2017;9:eaan1589.
- Gayet RV, et al. Creating CRISPR-responsive smart materials for diagnostics and programmable cargo release. Nat Protoc. 2020;15:3030-3063.







