ABOVE: Since the invention of the PASSIGE system, prime editing can tackle mutations from single base substitutions to structural variants. © istock.com, cdascher
CRISPR is perhaps the most popular tool in the genome editing toolkit. However, despite CRISPR’s obvious success, there are concerns about double-stranded breaks leading to unwanted, potentially dangerous edits.1 At the same time, some applications might require larger changes than the fairly limited single-base substitution repertoire of base editors. Prime editing, which can achieve targeted edits with single-nucleotide precision and without cutting both strands of DNA, may offer a solution.
Since the inventors Andrew Anzalone and David Liu published their first report in 2019,2 they have developed a next-generation system, started a company, and released the first encouraging preclinical data. Now, prime editing is finally heading towards the ultimate goal: therapy.
How prime editing works
Prime editing uses specialized machinery consisting of a prime editing guide RNA (pegRNA) and a Cas9 enzyme fused to a reverse transcriptase. The version of Cas9 used in prime editing has one of its two nuclease domains deactivated, turning it into a “nickase” that will only cut one of the DNA strands, rather than both. So, once the pegRNA binds to the right part of the DNA, the Cas9 nickase cuts the strand and creates a DNA “flap.”
The other end of the pegRNA acts as a template; part of it complements the flap, and another part bears the desired edit. The flap binds to a matching site, and the reverse transcriptase adds in the desired edit. The edited flap is incorporated back into the DNA, and with the help of a second nick on the complementary strand, DNA repair mechanisms fix the other strand to match the edit.
I don’t think prime editing needs to wait.
—Fyodor Urnov, University of California, Berkeley
While working in Liu’s lab at the Broad Institute, Anzalone achieved promising gene editing results in vitro, but his initial tests in human cells failed. He suspected that this might be due to the length of the flap binding site on the pegRNA. In one test, he happened to use a sequence that was just one nucleotide longer than the one he had used in vitro. “That one seemed like it had a trace above background editing,” he said. When he finally got positive gene editing results in human cells in early 2019, he knew the method had promise.
He repeated the experiments with a longer binding sequence. “I was in the lab—I think it was a weekend day—and there were some people in the office at the Broad where I was. They were looking over my shoulder as we processed the data and started seeing the first editing numbers in the single digits of percent of cells edited, but still very, very significantly above background,” he said. “That was a pretty exciting day.”
Fyodor Urnov, a biotechnologist at the University of California, Berkeley, reviewed the paper describing prime editing for Nature. “What struck me more than anything was what a marvelous extension it was of the theme that it represents,” he said. “When we want to do something to mother nature, we shouldn’t fight with her; we should collaborate with her.”
Twin Prime: the next-generation of prime editors
The original prime editor focused on smaller mutations, from single base substitutions to insertions and deletions tens of base pairs long, but it couldn’t fix longer sequences such as disease-causing structural variants. Consequently, Anzalone, Liu, and their colleagues came up with twin prime editing,3 named after the Twin Prime Conjecture in mathematics, which states that just like there is an infinite number of prime numbers, there is an infinite number of twin primes, like 17 and 19, that are prime numbers just two apart. One of Anzalone’s colleagues in the lab overlapped with the math department. “I thought it was a fun take,” he said.
For twin prime editing, Anzalone and Liu used two pegRNAs. By creating two flaps that overlap in the middle, they could edit a larger stretch of DNA by having one pegRNA edit the first half and the other edit the second half.
Twin prime editing, however, still struggled with inserting DNA larger than 100 base pairs. To go beyond that limit, the team added an extra element: site-specific recombinases. In nature, these enzymes recognize and bind to specific sites in DNA, cut the strands, and rearrange large segments before joining the strands back together. For Anzalone and Liu’s team, the potential for using them in genome editing seemed a perfect fit for boosting the insertion capacity of twin prime editing.
They decided to use twin prime editing to add recognition sites for the enzyme, which then integrates a piece of donor DNA. Prime Medicine, the company that Anzalone and Liu formed and where Anzalone now works, calls this bulked-up twin prime editing system PASSIGE, for prime assisted site specific integrase gene editing. “It could potentially enable gene-size integrations,” said Anzalone.
With PASSIGE, Anzalone and Liu have created a suite of prime editors that can tackle both small, medium, and large edits. The next obvious step, and the main goal of Prime Medicine, is to find out how these tools can be used to develop new gene therapies.
THE STEPS OF PRIME EDITING | |||
 | The prime editing machinery comprises a prime editing guide RNA (pegRNA) and a Cas9 nickase enzyme fused to a reverse transcriptase. | 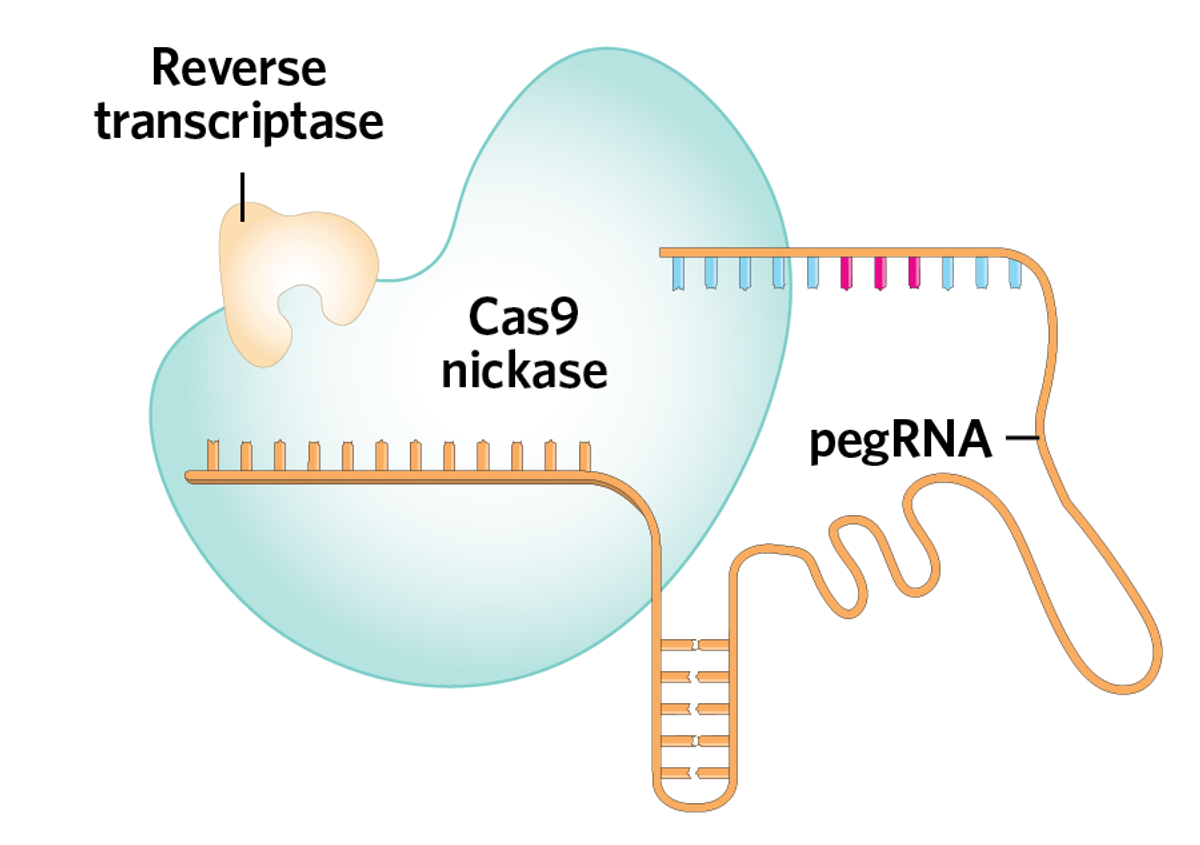 | |
 | |||
 | The pegRNA binds to a matching sequence on the target DNA strand. |  | The Cas9 nickase cuts the unbound complementary strand, creating a flap. |
 | |||
 | The dangling DNA flap binds to a matching sequence on the other end of the pegRNA. |  | The reverse transcriptase extends the flap by filling in the desired insertion sequence from the template next to the binding site. |
 | |||
 | The edited flap competes with and displaces the flanking nucleotide sequence (right), which is ultimately removed by the cell. |  | The opposite strand is nicked and repaired to match the newly edited flap, completing the edit. |
The promise of prime editing therapies
Despite now having several prime editing systems, the process of developing prime editing for therapy is potentially knottier than with other genome editing tools. “It’s not as simple a system as the original CRISPR-Cas9 or base editing where you have that single guide RNA and you target the site,” Anzalone said. “With prime editing, there are all these different tweaks and changes to the guide sequence, and that can make a really big difference on how efficient the editing is.”
While some see editing efficiency as a challenge still to be solved for prime editing,4 it hasn’t stopped Prime Medicine from pursuing several disease indications. They are furthest along with their ex vivo program focused on eventually treating chronic granulomatous disease, a genetic disorder in which people struggle to fight off certain infections due to impaired phagocytes. “We have some very encouraging preclinical results,” Anzalone said.
Anzalone believes that this is just the beginning. “Beyond correcting point mutations, we’re exploring repeat expansion diseases like Huntington’s disease and Friedreich’s ataxia where we believe we can remove those pathogenic repeats and potentially have an effect for patient populations,” he said. He also mentioned that prime editing can be used to generate potent antitumor CAR-T cells. “And that’s not even an indication in our pipeline right now.”
Urnov is excited by this progress but thinks that the most pressing task for Prime Medicine is to bring their technology to patients as soon as possible. “What prime editing needs to do is take the pipeline that they’ve described and aggressively advance one or two disease indications using the technology they have today,” he said.
He wouldn’t like to see Anzalone and his team focus on developing the technology to perfection instead of converting what they have into something that could help patients sooner. “The idea that you can optimize a technology for potency and safety until it’s clinic ready is just not the right mindset for our field,” he said. If regulatory standards are met, and there is transparency with patients, in his view, that’s enough. “I don’t think prime editing needs to wait.”
Anzalone hopes that Prime Medicine can achieve both goals. One way he envisions eventually maximizing the benefit of prime editing is something he calls “marching up the chromosome,” essentially addressing one mutation in a patient population that is more common, then a second, then a third, hopefully with a regulatory solution that won’t require a new process for each new pegRNA. Another would be to develop “long flap” systems that could fix all of the mutations in a particular exon that might be found in a patient population. “One drug product could treat a lot of patients, not just one,” he said.
It will take time for these ideas to come to fruition, time in which he will continue to push for the therapies he and his team are already developing. But it shows how difficult prioritization and strategy can be with a new technology. Which disease do you pick? Who gets to go first? Such questions rarely have easy answers.
“For early biotech, the biggest struggle is figuring out how many of those things you should do,” Anzalone said. “Everyone here wants to see [all of these things] happen. If we can’t get it to those places, it would be a shame.”
References
- Kosicki M, et al. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36(8):765-771.
- Anzalone AV, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149-157.
- Anzalone AV, et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol. 2022;40(5):731-740.
- Zhao Z, et al. Prime editing: advances and therapeutic applications. Trends Biotechnol. 2023.






