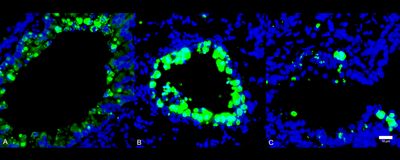
ABOVE: Comparison of immune cell infiltration (green) into lung tissue during SARS-CoV-2 infection in healthy (A), diabetic (B), and 1,5-AG–treated diabetic (C) mice. Modified from Fig. 4G in Nat Metab, DOI: 10.1038/s42255-022-00567-z, 2022.
Early on during the COVID-19 pandemic, experts noticed that people with diabetes were more likely to become hospitalized with the disease—the big question was why. Now, in vitro and in vivo work suggests that low concentrations of the metabolite 1,5-anhydro-D-glucitol (1,5-AG) may help explain the increased vulnerability of this population.
Researchers reported last week (May 9) in Nature Metabolism that 1,5-AG, a monosaccharide used as a blood biomarker for human diabetes mellitus because levels of the sugar are significantly lower in people with diabetes, binds to the spike protein of SARS-CoV-2 and prevents the virus from fusing with human cells—the crucial first step in cell entry. Moreover, supplementing diabetic mice with the sugar reduced COVID-19 severity, suggesting the molecule might hold promise as a treatment for the infection, particularly in people with diabetes.
Barry Rouse, an immunologist at the University of Tennessee, Knoxville, who did not participate in this study, says the data seem pretty convincing, and the concept of such a molecule interacting with a virus in this way is fascinating. Though, he says he is skeptical about the immediate clinical applicability of the findings.
Connecting diabetes to COVID-19 severity
Gong Cheng, an infectious disease researcher at the Tsinghua University School of Medicine in Beijing, China, and leader of the study, writes in an email to The Scientist that he and his colleagues were interested in understanding why there are “highly heterogeneous clinical manifestations in humans after the infection.” A lot of prior studies have mainly focused on differences “of genetic background or immunity,” he adds, but the team decided to study how the large metabolic variation among individuals may influence the clinical outcome of those infected.
They first generated a list of metabolites of interest by pooling the metabolic compounds in serum samples from three healthy patients with published metabolite profiles from SARS-CoV-2–infected individuals and uninfected volunteers. Among the 484 total metabolites identified, 222 were commercially available, which allowed Cheng and colleagues to test whether incubating cells with them impaired SARS-CoV-2 infection. Seven of them did, indeed, reduce the virus’s ability to infect cells.
The team then focused on one of the seven: 1,5-AG. Cheng explains that the small molecule is normally filtered into the kidney and later reabsorbed into the blood by a glucose cotransporter. However, because of the transporter’s affinity with glucose, 1,5-AG reabsorption can be “competitively inhibited by glucose,” he writes, which is high in the urine of people with diabetes. Thus, diabetic patients excrete more 1,5-AG in their urine resulting in lower levels of the metabolite in their blood.
In the team’s experiments, a much higher amount of SARS-CoV-2 RNA was detected after 24 hours of infection in cultured cells treated with sera from diabetic patients than that in cells treated with sera from healthy individuals. But supplementing the sera from diabetic donors with 1,5-AG reduced the amount of viral RNA produced in that time. The outcome was similar in a mouse model. Administering the metabolite to diabetic mice for ten days before infection with SARS-CoV-2 reduced the animals’ viral load and disease severity when measured three days after inoculation, compared with diabetic mice given a control solution.
When Cheng and colleagues explored what mechanisms could explain the metabolite’s impacts on the virus, they found that 1,5-AG binds to the S2 subunit of the SARS-CoV-2 spike protein, interrupting membrane fusion between the host cell and the virus.

The results suggest that administering 1,5-AG to COVID-19 patients with low levels of it, such as those with diabetes, could potentially improve clinical outcomes. However, the approach needs some fine-tuning, as administering the treatment to mice was already challenging. Cheng notes that supplementing the animals with 1,5-AG by intravenous, direct intratracheal, or oral administration was not very successful. “In all these approaches, the peak concentration of 1,5-AG appeared at 1–2 hours after administration and then rapidly [returned] to the basic physiological level in the serum and tissues, suggesting that 1,5-AG can be fast metabolized or released by animals,” he writes. Thus, for their experiments, they had to subcutaneously implant a pump in the animals that continuously provided them with the sugar.
Based on the limitations already seen in the mouse experiments, Rouse says supplementation in humans might not be “a practical approach to control the susceptibility of diabetics to COVID-19.”
Cheng adds that his team is currently developing 1,5-AG derivatives in the hopes of finding one that is effective in humans and testing it in clinical trials.